Abstract
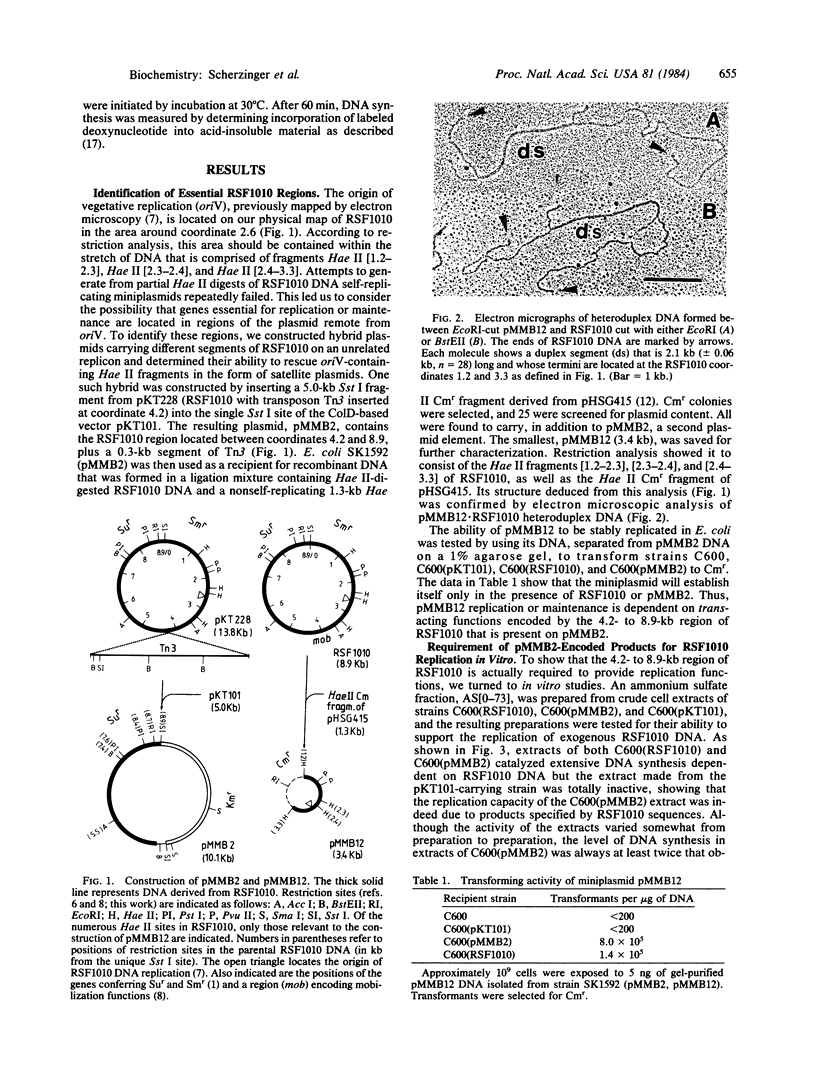
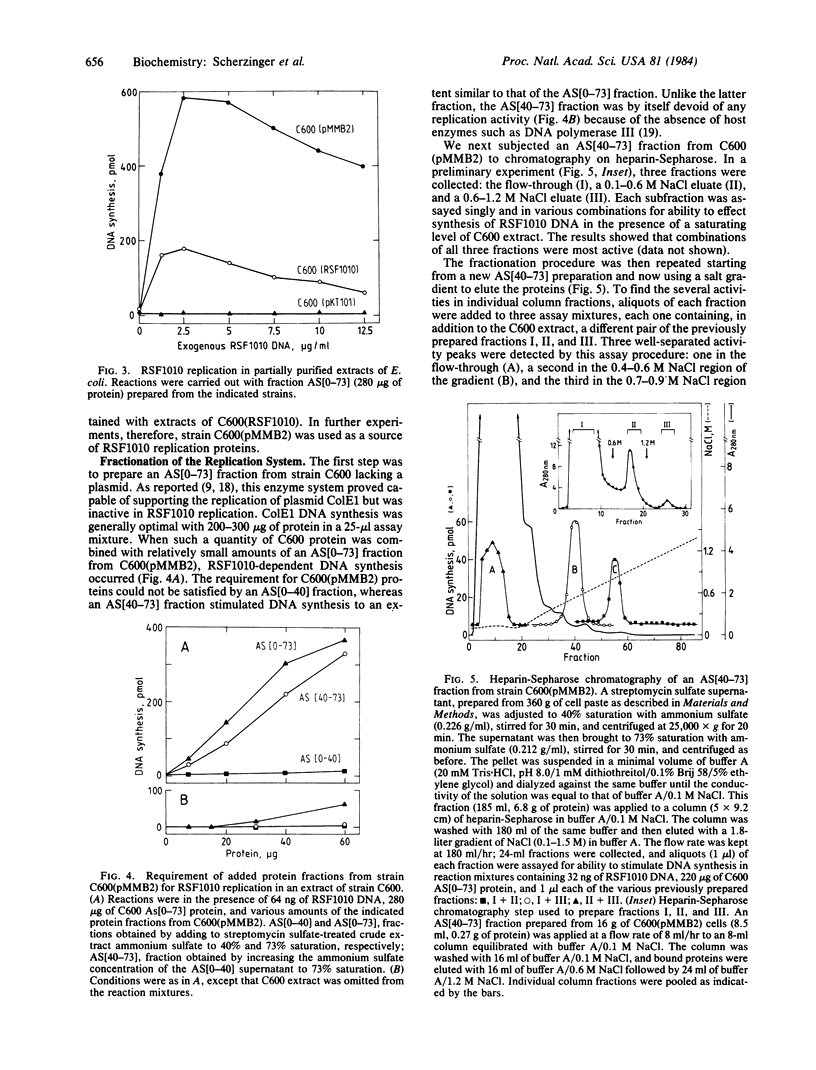
Cloning of specific regions of plasmid RSF1010, in conjunction with in vitro replication studies, has revealed three novel genes: repA, repB, and repC. They are clustered in one region of the plasmid, separated from the origin of replication by regions that are not essential for plasmid viability in an Escherichia coli host. In vivo, a 2.1-kilobase segment of the plasmid, bearing the replication origin, can establish itself as an autonomous replicon if the DNA region carrying the three rep genes is present in the same cell on an independent plasmid. In vitro, RSF1010 DNA is efficiently replicated by an ammonium sulfate fraction from the E. coli extract, provided the extracts are prepared from cells that can supply the required rep gene products. Using cells containing the cloned rep gene region as a source of elevated levels of the rep proteins, we have partially purified these proteins in functional form. When added to an enzyme fraction derived from plasmid-free cells, they specifically promote the replication of plasmid DNA bearing the RSF1010 origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrés I., Slocombe P. M., Cabello F., Timmis J. K., Lurz R., Burkardt H. J., Timmis K. N. Plasmid replication functions. II. Cloning analysis of the repA replication region of antibiotic resistance plasmid R6-5. Mol Gen Genet. 1979 Jan 5;168(1):1–25. doi: 10.1007/BF00267929. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Betlach M., Boyer H. W., Yanofsky S. Genetic and physical studies on the replication of ColE1-type plasmids. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):69–76. doi: 10.1101/sqb.1979.043.01.012. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Timmis K. N. Host: vector systems for gene cloning in Pseudomonas. Curr Top Microbiol Immunol. 1982;96:47–67. doi: 10.1007/978-3-642-68315-2_4. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Yoshinaga K., Lother H., Messer W. Purification of the E. coli dnaA gene product. EMBO J. 1982;1(12):1545–1549. doi: 10.1002/j.1460-2075.1982.tb01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Diaz R., Staudenbauer W. L. Replication of the broad host range plasmid RSF1010 in cell-free extracts of Escherichia coli and Pseudomonas aeruginosa. Nucleic Acids Res. 1982 Aug 11;10(15):4687–4702. doi: 10.1093/nar/10.15.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin F. C., Bagdasarian M., Bagdasarian M. M., Timmis K. N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Rückert B., Lurz R., Bister K. Two unrelated cell-derived sequences in the genome of avian leukemia and carcinoma inducing retrovirus MH2. EMBO J. 1983;2(11):1969–1975. doi: 10.1002/j.1460-2075.1983.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai N., Nishizawa T., Hayakawa Y., Murotsu T., Matsubara K. Detection and mapping of six miniF-encoded proteins by cloning analysis of dissected miniF segments. Mol Gen Genet. 1982;186(2):193–203. doi: 10.1007/BF00331850. [DOI] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light J., Molin S. Replication control functions of plasmid R1 act as inhibitors of expression of a gene required for replication. Mol Gen Genet. 1981;184(1):56–61. doi: 10.1007/BF00271195. [DOI] [PubMed] [Google Scholar]
- Linder P., Churchward G., Caro L. Plasmid pSC101 replication mutants generated by insertion of the transposon Tn1000. J Mol Biol. 1983 Oct 25;170(2):287–303. doi: 10.1016/s0022-2836(83)80149-1. [DOI] [PubMed] [Google Scholar]
- Meyer R., Hinds M., Brasch M. Properties of R1162, a broad-host-range, high-copy-number plasmid. J Bacteriol. 1982 May;150(2):552–562. doi: 10.1128/jb.150.2.552-562.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Schuster H., Mikolajczyk M., Rohrschneider J., Geschke B. phiX174 DNA-dependent DNA synthesis in vitro: requirement for P1 ban protein in dnaB mutant extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3907–3911. doi: 10.1073/pnas.72.10.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Kolter R., Helinski D. R. Plasmid R6K DNA replication. I. Complete nucleotide sequence of an autonomously replicating segment. J Mol Biol. 1982 Oct 15;161(1):33–43. doi: 10.1016/0022-2836(82)90276-5. [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Meyer R., Helinski D. R. Regions of broad-host-range plasmid RK2 which are essential for replication and maintenance. J Bacteriol. 1980 Jan;141(1):213–222. doi: 10.1128/jb.141.1.213-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Danbara H., Brady G., Lurz R. Inheritance functions of group IncFII transmissible antibiotic resistance plasmids. Plasmid. 1981 Jan;5(1):53–75. doi: 10.1016/0147-619x(81)90077-9. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Selzer G. Initiation of DNA synthesis in Escherichia coli. Annu Rev Biochem. 1979;48:999–1034. doi: 10.1146/annurev.bi.48.070179.005031. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Replication of bacteriophage lambda DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):681–691. doi: 10.1101/sqb.1983.047.01.079. [DOI] [PubMed] [Google Scholar]
- de Graaff J., Crosa J. H., Heffron F., Falkow S. Replication of the nonconjugative plasmid RSF1010 in Escherichia coli K-12. J Bacteriol. 1978 Jun;134(3):1117–1122. doi: 10.1128/jb.134.3.1117-1122.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



