Abstract
Background
SCIPIO is a first-in-human, phase 1, randomized, open-label trial of autologous c-kit+ cardiac stem cells (CSCs) in patients with heart failure of ischemic etiology undergoing coronary artery bypass grafting (CABG). Here, we report the surgical aspects and interim cardiac magnetic resonance (CMR) results.
Methods and Results
A total of 33 patients (20 CSC-treated and 13 controls) met final eligibility criteria and were enrolled in SCIPIO. CSCs were isolated from the right atrial appendage harvested and processed during surgery. Harvesting did not affect cardiopulmonary bypass, cross-clamp, or surgical times. In CSC-treated patients, CMR showed a marked increase in both LVEF (from 27.5 ± 1.6% to 35.1 ± 2.4 % [P=0.004, n=8] and 41.2 ± 4.5 % [P=0.013, n=5] at 4 and 12 months after CSC infusion, respectively) and regional EF in the CSC-infused territory. Infarct size (late gadolinium enhancement) decreased after CSC infusion (by manual delineation: -6.9 ± 1.5 g [-22.7%] at 4 months [P=0.002, n=9] and -9.8 ± 3.5 g [-30.2%] at 12 months [P=0.039, n=6],). LV non-viable mass decreased even more (-11.9 ± 2.5 g [-49.7%] at 4 months [P=0.001] and -14.7 ± 3.9 g [-58.6%] at 12 months [P=0.013]), while LV viable mass increased (+11.6 ± 5.1 g at 4 months after CSC infusion [P=0.055] and +31.5 ± 11.0 g at 12 months [P=0.035]).
Conclusions
Isolation of CSCs from cardiac tissue obtained in the operating room is feasible and does not alter practices during CABG surgery. CMR shows that CSC infusion produces a striking improvement in both global and regional LV function, a reduction in infarct size, and an increase in viable tissue, which persist at least 1 year and are consistent with cardiac regeneration.
Clinical Trial Registration
This study is registered with clinicaltrials.gov, trial number NCT00474461.
Keywords: Coronary artery bypass, heart failure, infarction, magnetic resonance imaging, stem cells, regeneration
Introduction
The prevalence of heart failure (HF) in industrialized nations has reached epidemic proportions; in the U.S., it is nearly 6 million and continues to rise. Despite notable advances, the prognosis of patients who are hospitalized with HF remains poor, with a 5-year mortality that approaches 50%1. Ischemic heart disease, with the attendant loss of myocardium, is considered to be the most common underlying cause of HF2-4. Consequently, the concept of regenerating myocardium with stem cell therapy has garnered increasing attention amongst investigators within the cardiovascular research community. Most efforts made to date have focused on bone marrow-derived progenitor cells5. In the study reported herein, we used a different approach that harnesses stem cells normally found in the heart itself.
Anversa and colleagues were the first to describe the existence of cardiac stem cells (CSCs), a resident population of stem cells in adult mammalian myocardium that is marked by the surface receptor tyrosine kinase c-kit.6 Numerous preclinical studies have demonstrated the efficacy of this cell population in the treatment of HF secondary to myocardial infarction (MI)6-8. This large body of preclinical evidence motivated us to undertake the first-in-human clinical trial of CSCs9. In August 2008, we received approval from the Food and Drug Administration to conduct SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy), a phase 1 clinical trial of CSCs. The primary goal of SCIPIO is to investigate the safety and feasibility of using autologous CSCs for the treatment of HF resulting from ischemic heart disease. The secondary goal is to gain initial insights into the effects of CSCs on left ventricular (LV) function, infarct size, and functional status. Since SCIPIO is the first study of CSCs in humans, the results will be important for developing this new form of cell therapy and planning future studies.
An interim analysis of SCIPIO has recently been published, and has shown a significant increase in LV ejection fraction (EF), measured by 3D echocardiography, in functional capacity, assessed by NYHA class, and in quality of life, gauged by the Minnesota Living With Heart Failure Questionnaire 9. That analysis, however, was limited by the fact that LVEF was not assessed by cardiac magnetic resonance (CMR), which is considered the “gold standard” for measuring LV function and infarct size10-14, that infarct size was not measured by semi-automated methods, that viable LV mass was not assessed, and that the surgical aspects of the study were not evaluated.
Since that initial report,9 we have completed enrollment of control and treated subjects and performed a detailed CMR analysis. Accordingly, we describe here the surgical aspects of the entire cohort of control and treated patients enrolled in SCIPIO. Additionally, we present the interim results of a comprehensive CMR evaluation of global and regional LV function, infarct size, and LV viable tissue.
Methods
Initial and Final Enrollment (Before and After Coronary Artery Bypass Grafting [CABG])
SCIPIO is a first-in-human, phase 1, randomized, open-label clinical trial designed to explore the effects of autologous c-kit-positive CSCs in patients with ischemic cardiomyopathy. The trial is still ongoing. A full description of the study protocol and inclusion and exclusion criteria has been published9; enrollment and exclusions are summarized in Fig. 1.
Figure 1.
Screening and enrollment as of November 12, 2011. CABG = coronary artery bypass graft surgery, LVEF = left ventricular ejection fraction, CSC = cardiac stem cell. * patient excluded from analysis because baseline cMRI was not performed due to newly placed thoracic staples.† patient excluded from analysis because of refusal to undergo baseline cMRI. ** The majority of those who withdrew refused to follow the rigorous testing regimen schedule set forth for all patients regardless of treatment allocation.
In summary, criteria for inclusion were HF with an LVEF ≤ 40%, evidence of a previous myocardial infarction (MI), and need for CABG. Final enrollment was based on assessment for eligibility at two time points. If a patient met the initial enrollment criteria within 2 weeks of CABG surgery, and agreed to participate, he/she signed an Institutional Review Board-approved informed consent prior to tissue harvest.. The patient was then reevaluated at 4 months after CABG surgery (Fig. 1); if the eligibility criteria were met, and the patient agreed to continued participation, the patient was included in the study. An interval of 4 months from surgery to cell therapy was selected because, in many patients with low LVEF, this variable is known to improve spontaneously during the first few months after CABG surgery as a result of resolution of myocardial hibernation and/or stunning15, 16; since this spontaneous improvement usually occurs within the first 4 months, administering cell therapy after a 4-month interval enabled us to separate the effects of CSCs from those of surgical revascularization17-19.
The trial was conducted in two sequential stages (Fig. 1). Stage A, aimed mainly at assessing feasibility and short-term safety, proceeded with the consecutive enrollment of 9 treated patients followed by 4 control patients. Subsequently, in stage B, an adaptive block randomization scheme was employed as previously described. Using this template, 11 and 9 patients were assigned to final enrollment in the treated and control groups, respectively 9. Thus, while 98 patients were enrolled based on satisfaction of initial enrollment eligibility (Fig. 1), only 33 patients (20 CSC-treated and 13 controls) met final eligibility criteria and agreed to participate in the study. Data regarding safety herein are reported for all 33 patients, all of whom will be included in the final analysis of data collected at pre-specified time points; there have been no patients lost to follow-up. The study protocol was approved by the Institutional Review Board of the participating clinical centers and the trial results were monitored by an independent Data and Safety Monitoring Board.
Surgical Methods and Cell Production
C-kit-positive cells were isolated and grown from the right atrial appendage. Consequently, only patients undergoing ”on-pump” CABG surgery were enrolled in the trial, as the appendage is routinely removed in these cases to allow access for right atrial cannulation. This tissue sample (~1 g) was excised and placed into a sterile specimen cup, which was then passed on to an investigator in the operating room. Using a sterile field (Supplemental Fig. 1) and aseptic technique, the investigator transferred the specimen to a sterile Petri dish, where the tissue was immersed in CSC growth medium. The tissue was minced into fine fragments (~ 200-400 μg each), which were placed into several sterile cryovials (Nalgene Nunc International, Rochester, NY, USA) filled with freezing solution (growth medium and DMSO in a 9:1 ratio). The cryovials were transported on ice to a Good Manufacturing Practice laboratory (Brown Cancer Center, University of Louisville) for storage at -80°C. Cryopreservation in a specialized cryovial ensured slow cooling (approximately -1°C per hour), thereby preserving cell viability. After ~80 h, the tissue was shipped overnight, via courier, in a freezing container to the core laboratory at the Brigham and Women's Hospital for dissociation, isolation, and expansion of CSCs. Once allocation to the control group was confirmed, those cell cultures were destroyed in compliance with institutional biohazard handling policies. The methods for expanding CSCs from the atrial tissue have been described previously9.
Preparation for CSC Infusion
At ~ 4 months (range, 3 to 5 months) after surgery, patients allocated to the treated arm returned for pre-treatment evaluation, which included 2D/3D echocardiography and CMR. CMR studies were performed only if renal function was adequate (eGFR>40 mg/ml) and if no standard CMR contraindications existed (e.g. prior ICD/pacemaker, metal hardware, etc.). Decisions regarding which vascular territory should be infused with CSCs were made by a group of investigators that included an interventional cardiologist, a radiologist, a noninvasive cardiologist, and a cardiac surgeon. Infusion territories were denoted by identifying infarcted region(s) using all of the imaging modalities available for each patient. The infusion vessel(s) was chosen based upon the anatomic association between the coronary artery and the infarcted region(s). If the territory to be infused was the anterior LV wall (supplied by the left anterior descending artery), 1 × 106 CSCs were injected into the graft supplying the left anterior descending artery. For other territories, 5 × 105 CSCs were injected into the graft(s) supplying those regions (Supplemental Table 1). If two infarcts were identified, 5 × 105 cells were infused into each infarcted territory. Regardless of number of infarcts or territories involved, a maximum of 1 × 106 CSCs was allowed for each patient. Cells were transported from the Brigham and Women's Hospital to the University of Louisville; the vial to be injected was prepared by suspending CSCs in Plasmalyte A solution.
CMR Studies
In patients eligible for CMR, these studies were performed at the University of Louisville using a 1.5T MR scanner (Espree, Siemens Medical Solutions, Erlangen, Germany). Screening and enrollment of patients for the CMR studies are summarized in Fig. 1. After anatomic axes of the heart were determined by scout images, a complete short-axis steady-state free precession (SSFP) cine sequence series covering the whole heart with 10-16 contiguous slices was obtained. The methods for acquisition and analysis of CMR images are described in the Supplemental section.
Statistical Analysis
The 33 eligible, evaluable patients were analyzed according to the treatment received (i.e., the analysis combined data from both stages of the study and does not follow the intention to treat paradigm for the patient who refused the experimental treatment). The CMR assessments were performed in a blinded fashion. All global and regional data were collected, after which segments treated with CSCs were unblinded for statistical analyses alone. Changes in EF and infarct size over time were assessed using repeated measures ANOVA followed by paired t tests. Correlations between two imaging modalities were initially analyzed using Pearson's Correlation. Those with significant correlation (P<0.05) were then further delineated for association using linear regression models. Data are reported as means ± SEM. All analyses were performed using SPSS 19.0 (SPSS, Inc., Chicago, IL).
Results
Surgical Aspects of SCIPIO
Enrollment in SCIPIO is complete (20 treated and 13 control patients). Of the 20 treated patients, 18 received 1 × 106 cells whereas two received 5 × 105cells (Supplemental Table 1). Seventy-five percent of the surgical grafts used for cell infusion were venous.
The baseline characteristics of enrolled subjects are summarized in Table 1. Important clinical variables that may affect surgical outcome, such as diabetes, hypertension, body mass index, and serum creatinine, were not statistically different between the treated and control groups. There were no differences in demographic information such as age, race, and gender. Measures of pre-operative cardiac status, such as number of diseased coronary vessels, location of infarction, and infarct-related arteries, were similarly distributed between the two groups.
Table 1.
Baseline Characteristics
| Treated (n = 20) | Control (n = 13) | P | |
|---|---|---|---|
| Age | 57.6 (1.9) | 55.8 (2.5) | 0.565 |
| Race | |||
| Caucasian | 19 (95%) | 12 (92%) | 0.693 |
| African american | 1 (5%) | 1 (8%) | |
| Male sex | 18 (90%) | 12 (92%) | 0.667 |
| Body mass index (kg/m2) | 29.7 (1.0) | 27.7 (1.3) | 0.210 |
| Diabetes mellitus | 5 (25%) | 5 (38%) | 0.664 |
| Hypertension | 17(85%) | 9(69%) | 0.393 |
| Hyperlipidemia | 14 (70%) | 11 (85%) | 0.431 |
| Tobacco use | 5 (25%) | 6 (46%) | 0.270 |
| Positive family history of CAD | 9 (45%) | 7 (54%) | 0.888 |
| Baseline ejection fraction | 29.9 (1.7) | 29.2 (1.9) | 0.796 |
| Number of arteries with stenosis >50% | 2.9 (0.1) | 2.6 (0.1) | 0.187 |
| Infarct Artery | |||
| RCA | 17 (47%) | 10 (43%) | |
| LAD | 15 (42%) | 11 (48%) | |
| LCx | 4 (11%) | 2 (9%) | |
| Number of old infarcts | 1.8 (0.1) | 1.8 (0.2) | 0.862 |
| Anterior infarction | 15 (42%) | 11 (48%) | |
| Non-anterior infarction | 21 (58%) | 12 (52%) | |
| Number of vessels infused | 1.7 (0.1) | N/A | N/A |
| LIMA | 8 (24%) | N/A | |
| Non-LIMA | 25 (76%) | N/A | |
| Number of cells injected | |||
| 1,000,000 | 18 (90%) | N/A | N/A |
| 500,000 | 2 (10%) | N/A | N/A |
| Medications | |||
| Aspirin | 20 (100%) | 12 (92%) | 0.826 |
| β Blocker | 16(80%) | 12 (92%) | 0.641 |
| ACE inhibitors or ARB | 14 (70%) | 7 (54%) | 0.567 |
| Statin | 17 (85%) | 11 (85%) | 0.641 |
| Clopidogrel | 7 (35%) | 5 (39%) | 0.866 |
Baseline characteristics of all patients enrolled in the SCIPIO trial. Data are number (%) or mean ± SEM. N/A=not applicable, CAD=coronary artery disease, RCA=right coronary artery, LAD=left anterior descending, LCx=left circumflex, LIMA=left internal mammary artery.
CABG surgery was performed at the clinical site of enrollment, either in Louisville (University of Louisville or Jewish Hospital) or in Chicago (Advocate Christ Medical Center). The surgical characteristics are summarized in Table 2. In general, this was a population of patients with severe HF. There were no statistically significant differences between control and treated groups with respect to the number of grafts placed and/or in the choice of arterial versus vein grafts. Four patients in each group were found to have significant left main coronary artery disease (stenosis >50%). Intra-aortic balloon pump placement was necessary in five CSC-treated and four control patients (this was done preoperatively in one control patient, post-operatively in another control, and intra-operatively in the other seven patients [Table 2]). Pre-operative LVEF was similar in the treated and control groups (25.3 ± 1.7% versus 27.8 ± 2.3%, P=.380). The proportion of patients with pre-operative LVEF ≤ 20% was 35% in the treated group and 31% in the control group. Concomitant valve surgery and number of repeat surgical procedures were similar between groups. As a result of these similarities, the Society of Thoracic Surgeons (STS) risk score for mortality or morbidity was not statistically different between groups (Table 2). Thus, the two groups of patients enrolled in SCIPIO were well-balanced with respect to important pre-operative predictors of outcome, supporting the concept that the improvement in LV function was due to CSC administration rather than baseline differences.
Table 2.
Surgical characteristics
| Treatment Group (n=20) | Control Group (n=13) | P | |
|---|---|---|---|
| Total surgery time (min) | 211.7 (9.8) | 229.8 (12.2) | 0.256 |
| Total CPB time (min) | 86.2 (5.6) | 101.5 (7.9) | 0.116 |
| Cross-clamp time (min) | 58.6 (4.7) | 61.0 (6.3) | 0.755 |
| IABP placed | 5 (25%) | 4 (31%) | 0.971 |
| Number of grafts | 2.3 (0.2) | 2.7 (0.2) | 0.099 |
| Vein grafts | 34 (74%) | 24 (69%) | 0.780 |
| Arterial grafts | 12 (26%) | 11 (31%) | |
| Grafts with >50% narrowing 4 months post-op | |||
| Vein grafts | 6 (18%) | N/A | N/A |
| Arterial grafts | 0 | ||
| STS pre-operative risk | |||
| Mortality | 2.2 (0.4) | 2.7 (0.9) | 0.575 |
| Morbidity or mortality | 21.1 (2.4) | 22.5 (4.2) | 0.757 |
| Concomittant valve surgery | 1 (5%) | 3 (23%) | 0.276 |
| Repeat surgery | 2 (10%) | 0 | 0.508 |
| Perioperative complication* | 2 (10%) | 1 (8%) | 0.693 |
| Positive sterility test | |||
| Endotoxin** | 0 | N/A | N/A |
| Mycoplasma | 0 | N/A | |
| Anaerobic culture | 0 | N/A | |
| Aerobic Culture | 0 | N/A |
Surgical characteristics of the treated and control groups. Data are reported as numbers (%) or means ± SEM. CPB = cardiopulmonary bypass; IABP = intra-aortic balloon pump.
Infected sternal wires and cellulitis at vein harvest site in CSC-treated patients, persistent sternal pain post-operatively in control patient.
Sterility testing was performed on the CSC solution at two time points prior to infusion. All testing was negative for microbial contamination.
Average values for incision to closure, cardiopulmonary bypass, and aortic cross-clamp times in the two groups combined (218.8 ± 7.7, 92.2 ± 4.7, and 59.5 ± 3.7 min, respectively [Table 2]) were similar to the average times reported for all open-heart surgeries performed at the main surgical center (Jewish Hospital) over the previous 12 months, indicating that intraoperative tissue procurement did not adversely affect standard operating procedure or compromise patient safety. There was no microbial contamination of any of the cell cultures, as confirmed by rigorous sterility testing that included aerobic and anaerobic cultures maintained for 14 days and independent assays for both mycoplasma detection and endotoxin levels (Table 2). Mean endotoxin levels for all samples tested were well below the accepted standard (< 5 EU/kg). Collectively, these results demonstrate the safety and feasibility of harvesting and preparing tissue in the operating room.
Having completed enrollment, we report here, for the first time, data on the safety of intracoronary CSC infusion in the entire population of 20 treated patients enrolled in SCIPIO (our previous report 9 was limited to 16 patients). In this cohort of 20 treated patients, we experienced two complications during CSC infusion into a graft (LIMA dissection repaired with covered stent, and elevated cardiac enzymes after balloon inflation consistent with peri-procedural MI). Interestingly, CSC infusion in the treated group offered a unique insight into the condition of the bypass grafts at 4 months after operation; angiograms performed at the time of CSC infusion demonstrated > 50% stenosis in 18% of vein grafts and none of the arterial grafts (Table 2). Overall, major adverse cardiac event (MACE) rates are similar between treated and control groups (Supplemental Table 2).
CMR Analysis
According to the original protocol, CMR studies were performed only in CSC-treated patients. The selection of the patients with CMR studies is described in Fig. 1. Of a total of 20 patients enrolled in the treated arm, nine were not eligible for CMR; among the remaining 11 patients, baseline CMR studies could not be performed in two, one because of recently-placed surgical clips and another because of patient's scheduling conflicts. Consequently, the analysis of CMR data was carried out in the nine treated patients that have baseline and 4-month follow-up images. The baseline characteristics of the patients included in the CMR analysis are described in Supplemental Table 3. The average age of the infarcts in this cohort was 4.1 ± 1.0 years.
EF Analysis
One patient was excluded from the EF analysis because of moderate to severe aortic stenosis. In the remaining eight patients, EF was measured both in the entire left ventricle (global EF analysis) and in the infarcted regions that received CSC infusion (regional EF analysis).
Global EF analysis
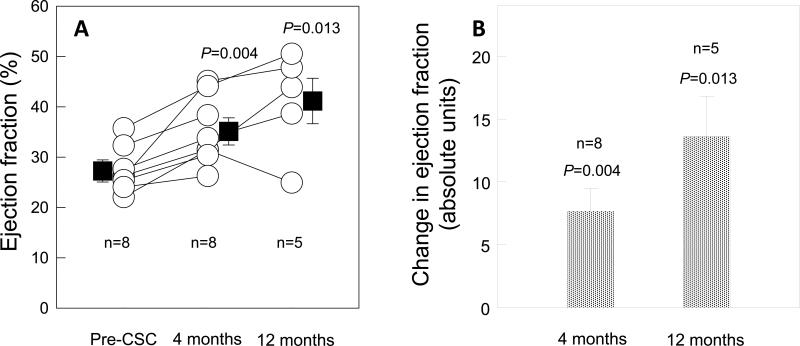
The mean baseline LVEF in the eight treated patients who were included in the CMR analysis was 27.5 ± 1.6% at baseline (4 months after CABG surgery and before CSC infusion), and increased markedly to 35.1 ± 2.4% (P=0.004, n=8) at 4 months and 41.2 ± 4.5% (P=0.013, n=5) at 12 months after CSC infusion (Fig. 2). By CMR, the net increase in LVEF was 7.7 EF units at 4 months (P=0.004) and 13.6 EF units at 12 months (P=0.013). Interestingly, strong correlation between measurements of LVEF by CMR and 3D echocardiography in this patient cohort was noted (Supplemental Fig. 2).
Figure 2.
Panel A: LVEF at baseline (27.5±1.6%), 4 months after CSC infusion (35.1± 2.4%), and 12 months after CSC infusion (41.2± 4.5%). Panel B: Change in LVEF at 4 months and 12 months after CSC infusion (absolute EF units). Data are means ± SEMs.
Regional EF analysis
In the infarcted regions that received intracoronary CSCs, regional EF averaged 10.3 ± 6.9 % at baseline (n=8); following CSC infusion, it increased by 14.2 EF units at 4 months (P=0.008, n=8) and by 17.9 EF units at 12 months (P=0.085, n=5) (Figs. 3 A and B). Of the eight patients with analyzable CMR studies, seven had dyskinetic segments. The mean regional EF of the dyskinetic segments was -20.6 ± 4.7% at baseline; after CSC infusion, it increased by 24.5 EF units at 4 months (P=0.014, n=7) and 35.7 units at 12 months (P=0.030, n=4) (Fig. 3 C and D). The mean regional EF of the each patient's least functional segment, which was -32.7 ± 9.8 % at baseline, increased by 25.6 EF units at 4 months (P=0.020, n=8) and 40.2 EF units at 12 months (P=0.023, n=5) (Fig. 3 E and F).
Figure 3.
Panel A: Regional EF at baseline and 4 and 12 months after CSC infusion in the infarct-related regions. Panel B: Change in regional EF in the infarct-related regions at 4 and 12 months after CSC infusion (absolute EF units). Panel C: Regional EF in the dyskinetic segments of the infarct-related regions at baseline and 4 and 12 months after CSC infusion. Panel D: Change in regional EF in the dyskinetic segments of the infarct-related regions at 4 and 12 months after CSC infusion (absolute EF units). Panel E: Regional EF in the least functional segment of the infarct-related regions at baseline and 4 and 12 months after CSC infusion. Panel F: Change in regional EF in the least functional segment of the infarct-related regions at 4 and 12 months after CSC infusion (absolute EF units). Data are means ± SEMs.
As illustrated in Fig. 3, the absolute improvement in regional EF in segments that were dyskinetic (Figs. 3D and F) was greater than that noted in the entire infarcted regions, which were, on average, hypokinetic (Fig. 3B). For example, at 1 year after CSC infusion, the regional EF of the least functional segments in the infarcted region increased by 40.2 ± 11.3 units (Fig. 3F), more than twice the average increase noted in the entire infarcted region (17.9 ± 7.9 units, Fig. 3B). These observations support the concept that the functional benefits of CSCs are inversely related to the baseline functional status of the myocardial region: the lower the baseline function, the greater the improvement afforded by CSC infusion.
Infarct Analysis
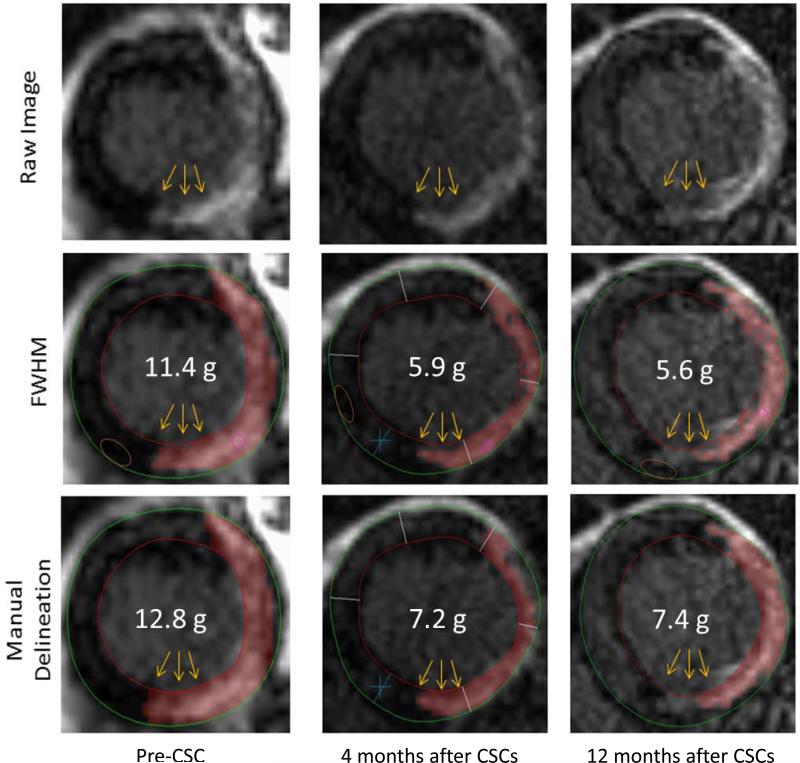
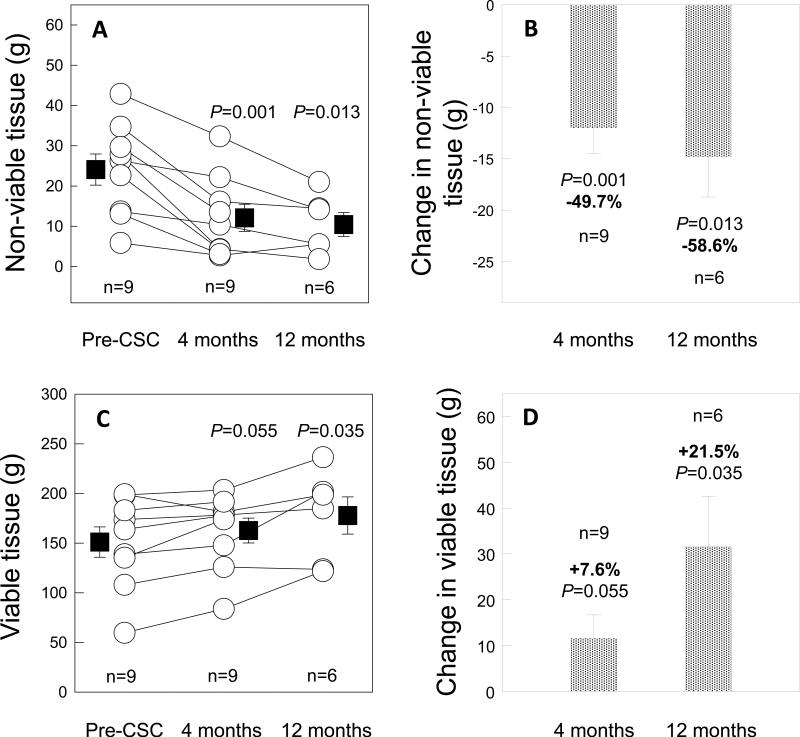
All of the nine treated patients that had CMR studies were included in the infarct analysis. An example of CMR images of an infarct before CSC infusion and 4 and 12 months after infusion is shown in Fig. 4. Infarct size, measured by manual delineation, averaged 30.4 ± 5.1 g at baseline and decreased to 23.5 ± 3.7 g at 4 months after CSC infusion (P=0.002 n=9) and 22.6 ± 5.5 g at 12 months (P=0.039, n=6), corresponding to a percent reduction of 22.7% and 30.2%, respectively (Figs. 5A and 5B). Measurement of infarct size by a semi-automated method (FWHM, which measures “core” infarct size [Supplemental Table 4]) yielded even more striking results: infarct size decreased from 34.9 ± 2.3 g at baseline to 21.6 ± 2.7 g at 4 months (P<0.001, n=9) and 18.7 ± 3.6 g at 12 months (P=0.003, n=6), corresponding to a percent reduction of 38.1% and 44.8%, respectively (Figs. 5C and 5D). The most dramatic reductions were noted when we calculated non-viable LV mass (measured by semi-automated methods and defined as areas with delayed enhancement in which the infarct involves ≥50% of the LV wall thickness [Supplemental Table 4]). Non-viable LV mass, which averaged 24.1 ± 3.9 g at baseline, decreased by 50% at 4 months after CSC infusion (to 12.1 ± 3.4 g; P=0.001, n=9) and by 59% at 12 months (to 10.4 ± 3.0 g; P=0.013, n=6) (Figs. 6 A and B). Thus, all three parameters used to assess infarct size consistently exhibited a dramatic reduction after administration of CSCs, although the magnitude of the benefit differed depending on the method used.
Figure 4.
Example of changes in the size of an infarct from baseline (before CSC infusion) to 4 and 12 months after CSC infusion. The numbers (in white) denote the infarct size (in g) in the slice shown; CSC=cardiac stem cell, FWHM=“full-width half-maximum” method, which uses all areas with >50% of the maximal signal intensity to define the infarcted myocardium. Note the decrease in gadolinium enhancement in the inferolateral wall at 4 months after CSCs, which persisted at 12 months.
Figure 5.
Panel A: Infarct size (in g) at 4 and 12 months after CSC infusion measured by manual delineation. Panel B: Reduction in infarct size at 4 and 12 months after CSC infusion measured by manual delineation. Panel C: “Core” infarct size (in g) at 4 and 12 months after CSC infusion measured by the full-width half-maximum (FWHM) method. Panel D: Reduction in core infarct size at 4 and 12 months after CSC infusion measured by the FWHM method. Data are means ± SEMs.
Figure 6.
Changes in viable and non-viable LV mass (defined as segments with infarcts involving < or >50% of the LV wall thickness, respectively). Panel A: Non-viable mass (in g) at 4 and 12 months after CSC infusion. Panel B: Reduction in non-viable mass (in g) at 4 and 12 months after CSC infusion. Panel C: Viable mass (in g) at 4 and 12 months after CSC infusion. Panel D: Increase in viable mass (in g) at 4 and 12 months after CSC infusion. Data are means ± SEMs.
At the same time, the total viable LV mass increased from 151.0 ± 15.3 g at baseline to 162.6 ± 12.5 g at 4 months (P=0.055) and 177.8 ± 18.8 g at 12 months (P=0.035), corresponding to a percent increase of 7.6% and 21.5%, respectively (Fig. 6 C and D). Based on the decrease in infarct size, and assuming that 90% of the regenerated myocardium is composed by cardiomyocytes and the volume of differentiated myocytes is ~20,000 μm320, it can be estimated that ~294 × 106 and 415 × 106 cardiomyocytes were generated within the scar at 4 and 12 months, respectively.
Discussion
As the first trial of CSC therapy in humans, SCIPIO has the potential to open a new avenue in the treatment of patients with ischemic cardiomyopathy and severe HF21. This patient population, which is quite large, currently has a poor prognosis and limited options22. We have recently reported the interim results of SCIPIO, which indicate that intracoronary infusion of autologous CSCs results in improved LVEF (assessed by 3D echocardiography), functional capacity (assessed by the NYHA class), and quality of life (assessed by the Minnesota Living With Heart Failure Questionnaire) 9. Since that initial report, we have completed patient enrollment and performed further analysis of the CMR studies, which enables us to present herein a complete report of the surgical aspects of SCIPIO and a summary of the CMR data available to date.
Our salient findings can be summarized as follows: i) harvesting and processing the right atrial appendage in the operating room for subsequent isolation and expansion of CSCs was eminently feasible in all 33 patients enrolled in the study, yielding successful CSC cultures in each case despite the fact that these patients have severe coronary artery disease, severe HF, and multiple comorbidities; ii) this new method for obtaining autologous stem cells did not interfere with standard surgical procedures or with surgical outcomes, and thus appears to be feasible in most patients undergoing CABG; iii) CMR (generally considered the most accurate technique for these analyses 10-14) demonstrated that intracoronary infusion of autologous CSCs ~4 months after CABG surgery resulted in a striking increase in LVEF (+ 7.7 EF units) 4 months later and an even greater increase (+13.6 EF units) 12 months later, accompanied by a parallel increase in regional contractile function in the infarcted regions that were infused with CSCs; iv) this improvement in LV function was coupled with a concomitant decrease in infarct size, which was consistently observed with three different CMR methods (reductions at 4 and 12 months after CSC infusion, respectively: -22.7 % and -30.2 % by manual delineation, -38.1% and -44.8% by a semi-automated method [FWHM], and -49.7% and -58.6% using non-viable mass), and was accompanied by an increase in LV viable mass (+11.6 g at 4 months and +31.5 g at 12 months after CSC infusion), implying robust regeneration of myocardial tissue. Taken together, these results, obtained with the best methodology currently available (CMR), indicate that administration of autologous CSCs obtained from a surgical specimen is effective in improving LV function and promoting cardiac regeneration in patients with chronic ischemic cardiomyopathy.
While the improvement in LV function observed herein with CMR is consistent with our previous observations with 3D echocardiography9, it must be underscored that the present study provides several important new pieces of information. This is the first analysis of the surgical characteristics of the entire cohort of patients enrolled in SCIPIO (20 treated and 13 controls). The results show that harvesting and processing right atrial appendages in the operating room did not prolong cardiopulmonary bypass time, aortic cross-clamp time, or total surgical time. CSCs were successfully isolated and expanded from all of the 33 specimens in this cohort, with no microbial contamination in any of the cell cultures. To our knowledge, SCIPIO is the first clinical trial to utilize surgical specimens for the isolation of stem cell populations to be used in the treatment of ischemic HF. Our present results demonstrate that this method for obtaining CSCs is safe and feasible in virtually all patients, and thus is applicable on a widespread basis.
This manuscript is the first analysis of the effects of CSC infusion on LVEF measured by CMR. The results show a striking improvement at 4 months, which persisted and became even more pronounced at 12 months (Fig. 2). LVEF would not be expected to improve spontaneously in these patients, who have LV scars ~ 4 years old and severe HF (Supplemental Table 3). Indeed, a previous CMR study in this patient population (ischemic cardiomyopathy) has demonstrated that in non-viable regions (defined as in the present study) there was no improvement in function following coronary revascularization23. We also found an excellent correlation between measurements of LVEF by 3-D echocardiography and CMR (Supplemental Fig. 2) – a finding that is important because many patients with chronic ischemic cardiomyopathy are not eligible for CMR studies.
In our previous report 9, we provided CMR measurements of wall thickening in the entire left ventricle (in six patients at 4 months) but did not assess regional function; here, we have expanded this analysis greatly by providing measurements of regional EF in the territories infused with CSCs, in a larger patient cohort (eight patients at 4 months and five patients at 12 months), and also separately in dyskinetic segments (Fig. 3). Our data demonstrate a marked improvement in the function of the infarcted regions that were treated with CSCs (Fig. 3), which is consistent with our data on regression of infarction (vide infra). We further demonstrate that the beneficial effects of CSCs were more pronounced in the LV segments that exhibited the greatest degree of contractile dysfunction (Fig. 3) - a finding that is important for the selection of patients for CSC therapy as well as for the design of future trials.
Compared with our previous report 9 in which we measured infarct size only by manual delineation in 7 patients at 4 months and 6 patients at 12 months, here we present a much more comprehensive analysis of infarct size and viability. Rather than relying on one method, as is frequently done, we used three CMR methods: i) manual delineation of infarct size [which remains the method by which most semi-automated methods are compared24,25, 26(Fig. 5A and 5B)], ii) the FWHM method to measure core infarct size (Fig. 5C and 5D) (a newer semi-automated methodology that appears to be extremely promising24), and iii) a semi-automated method to measure non-viable tissue mass (Fig. 6). All three methods demonstrated a consistent and robust reduction in infarcted tissue at 4 months that persisted or became even more pronounced at 12 months (Figs. 5 and 6). The reduction, however, was more dramatic using the measurements of core infarct (-45% at 12 months) and non-viable mass (-59% at 12 months) than manual delineation (-30% at 12 months). By employing three separate approaches, we sought to strengthen the association of infarct regression with CSC therapy, particularly because controversies regarding infarct measurements by CMR have become more prevalent27 as several semi-automated methods have been recently described. The concordance of the three methods in demonstrating a decrease in infarcted tissue bolsters the robustness of the conclusions. The differences among the methods in the magnitude of infarct size reduction (Figs. 5 and 6) are interesting in and of themselves, as they point to the need to standardize infarct size measurements by CMR in the future.
Regardless of its exact magnitude, the finding of infarct regression is conceptually important. Since the infarcts in the patients undergoing CMR studies were, on average, ~ 4 years old (Supplemental Table 3), and since old myocardial scars are stable and do not shrink over time28, the decrease in infarct (scar) size and the replacement of scar with viable myocardium (Fig. 6) indicate myocardial regeneration. CABG is insufficient to account for our findings; indeed, previous studies have shown no benefit with revascularization (either reduction in infarct size or improvement in function) when infarcts involving >50% of the LV wall thickness (such as those present in our patients) were noted with CMR delayed enhancement13, 23. In fact, to our knowledge, no previous investigation has demonstrated a decrease in the size of an old infarct following coronary revascularization. In contrast, in SCIPIO the non-viable mass decreased strikingly (by 50% at 4 months after CSCs and by 59% at 12 months [Fig. 6A and 6B]), a finding that cannot be explained by the natural history of the disease or by revascularization. This notion is also supported by studies of bone marrow cell infusion in the setting of an old MI, in which patients enrolled in the control arm did not exhibit any significant changes in either LV function or infarct size over time29-31. Even in the setting of acute MI and subsequent revascularization, little or no change in myocardial function or scar size was found at 2 months after revascularization 32 and global LV function was reported to remain similar from 5 days to 1 year after MI33.
In addition to showing a decrease in infarct size, this is the first report to show that administration of CSCs results in an increase in viable tissue at 4 and 12 months (Fig. 6C and 6D). We applied known morphometric parameters 20 to estimate the number of new myocytes that had to be formed in order to account for the observed replacement of scar with viable tissue. Using, conservatively, the smallest of our three measures of infarct regression (Figs. 5 and 6), i.e., that obtained by manual delineation, we calculated that 294 × 106 and 415 × 106 cardiomyocytes were generated within the scar at 4 and 12 months, respectively. Our estimates of regeneration are consistent with previous observations in animal models of MI, in which the injection of human CSCs resulted in a similar recovery of cardiac muscle mass. 34, 35
An interesting finding was that the magnitude of functional improvement was greater in regions where the infarcts had greater transmurality (Supplemental Fig 3). Also, we found that the functional improvement was greater in dyskinetic segments than in the entire infarcted region (+24 and +36 EF units at 4 and 12 months, respectively, vs. +14 and +18 EF units in the entire infarct-related region; Fig. 3A, B, C, and D), and that the segment with the greatest level of dysfunction exhibited the greatest improvement following CSC infusion (+26 and +40 EF units at 4 and 12 months, respectively; Fig. 3E and 3F). These findings indicate that the functional improvement effected by CSCs is greatest in the regions that have the greatest level of baseline dysfunction – a concept that had not been appreciated before and will be important in the design of future trials of stem cell therapy. Conceptually, our results are consistent with those of the REPAIR-AMI MRI substudy, in which patients with more profound ventricular dysfunction received a significant benefit from administration of bone marrow mononuclear cells36; however, this is the first report that the improvement in regional function affected by stem cells is greatest in the segments with greatest baseline dysfunction.
In summary, the results presented herein demonstrate the safety and feasibility of isolating and expanding CSCs from cardiac tissue obtained during CABG surgery, a new method for procuring stem cells for the treatment of ischemic cardiomyopathy. Using CMR (the current “gold standard”), we demonstrate that CSCs obtained with this method effect a striking improvement in global and regional LV function, concomitant with a profound decrease in infarct size and an increase in viable tissue, indicative of cardiac regeneration. This new method for CSC procurement and therapy is potentially applicable to most patients undergoing CABG surgery and appears to be remarkably efficacious. Larger studies of CSCs isolated and expanded from surgical specimens are, therefore, warranted.
Supplementary Material
Acknowledgments
Funding Sources
Supported in part by NIH Grant R37HL081737.
Footnotes
Disclosures
PA is a member of Autologous. The other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics C. Stroke Statistics S Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: A report from the italian network on congestive heart failure. American heart journal. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 3.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: The health, aging, and body composition study. Archives of internal medicine. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in us men and women: Nhanes i epidemiologic follow-up study. Archives of internal medicine. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 6.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 7.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Soliman OI, Kirschbaum SW, van Dalen BM, van der Zwaan HB, Mahdavian Delavary B, Vletter WB, van Geuns RJ, Ten Cate FJ, Geleijnse ML. Accuracy and reproducibility of quantitation of left ventricular function by real-time three-dimensional echocardiography versus cardiac magnetic resonance. The American journal of cardiology. 2008;102:778–783. doi: 10.1016/j.amjcard.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 11.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 12.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed q-wave and non-q-wave myocardial infarction. Lancet. 2001;357:21–28. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. The New England journal of medicine. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 14.Hendel RC, Patel MR, Kramer CM, Poon M, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Douglas PS, Peterson ED, Wolk MJ, Allen JM. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Bax JJ, Visser FC, Poldermans D, Elhendy A, Cornel JH, Boersma E, Valkema R, Van Lingen A, Fioretti PM, Visser CA. Relationship between preoperative viability and postoperative improvement in lvef and heart failure symptoms. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2001;42:79–86. [PubMed] [Google Scholar]
- 16.Shivalkar B, Maes A, Borgers M, Ausma J, Scheys I, Nuyts J, Mortelmans L, Flameng W. Only hibernating myocardium invariably shows early recovery after coronary revascularization. Circulation. 1996;94:308–315. doi: 10.1161/01.cir.94.3.308. [DOI] [PubMed] [Google Scholar]
- 17.Vanoverschelde JL, Depre C, Gerber BL, Borgers M, Wijns W, Robert A, Dion R, Melin JA. Time course of functional recovery after coronary artery bypass graft surgery in patients with chronic left ventricular ischemic dysfunction. Am J Cardiol. 2000;85:1432–1439. doi: 10.1016/s0002-9149(00)00790-6. [DOI] [PubMed] [Google Scholar]
- 18.Lorusso R, La Canna G, Ceconi C, Borghetti V, Totaro P, Parrinello G, Coletti G, Minzioni G. Long-term results of coronary artery bypass grafting procedure in the presence of left ventricular dysfunction and hibernating myocardium. Eur J Cardiothorac Surg. 2001;20:937–948. doi: 10.1016/s1010-7940(01)00945-9. [DOI] [PubMed] [Google Scholar]
- 19.Mintz LJ, Ingels NB, Jr., Daughters GT, 2nd, Stinson EB, Alderman EL. Sequential studies of left ventricular function and wall motion after coronary arterial bypass surgery. Am J Cardiol. 1980;45:210–216. doi: 10.1016/0002-9149(80)90637-2. [DOI] [PubMed] [Google Scholar]
- 20.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 21.Heusch G. Scipio brings new momentum to cardiac cell therapy. Lancet. 2011;378:1827–1828. doi: 10.1016/S0140-6736(11)61648-6. [DOI] [PubMed] [Google Scholar]
- 22.Shah PJ, Hare DL, Raman JS, Gordon I, Chan RK, Horowitz JD, Rosalion A, Buxton BF. Survival after myocardial revascularization for ischemic cardiomyopathy: A prospective ten-year follow-up study. J Thorac Cardiovasc Surg. 2003;126:1320–1327. doi: 10.1016/s0022-5223(03)00809-2. [DOI] [PubMed] [Google Scholar]
- 23.Bove CM, DiMaria JM, Voros S, Conaway MR, Kramer CM. Dobutamine response and myocardial infarct transmurality: Functional improvement after coronary artery bypass grafting--initial experience. Radiology. 2006;240:835–841. doi: 10.1148/radiol.2403051150. [DOI] [PubMed] [Google Scholar]
- 24.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC. Cardiovascular imaging. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Hsu LY, Natanzon A, Kellman P, Hirsch GA, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement mri. Part i: Animal validation of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:298–308. doi: 10.1002/jmri.20496. [DOI] [PubMed] [Google Scholar]
- 26.Hsu LY, Ingkanisorn WP, Kellman P, Aletras AH, Arai AE. Quantitative myocardial infarction on delayed enhancement mri. Part ii: Clinical application of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging. 2006;23:309–314. doi: 10.1002/jmri.20495. [DOI] [PubMed] [Google Scholar]
- 27.Nacif MS, Chugh AR, Lima JA, Bluemke DA. How should infarct size be measured on lge sequences? A call for a change in the guidelines. JACC. Cardiovascular imaging. 2011;4:1223. doi: 10.1016/j.jcmg.2011.08.010. author reply 1224. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 29.Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heart failure: The star-heart study. Eur J Heart Fail. 2010;12:721–729. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]
- 30.Gao LR, Wang ZG, Zhu ZM, Fei YX, He S, Tian HT, Zhang NK, Chen Y, Xu HT, Yang Y. Effect of intracoronary transplantation of autologous bone marrow-derived mononuclear cells on outcomes of patients with refractory chronic heart failure secondary to ischemic cardiomyopathy. Am J Cardiol. 2006;98:597–602. doi: 10.1016/j.amjcard.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller HW, Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The iact study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 32.Orn S, Manhenke C, Greve OJ, Larsen AI, Bonarjee VV, Edvardsen T, Dickstein K. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J. 2009;30:1978–1985. doi: 10.1093/eurheartj/ehp219. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder AP, Houlind K, Pedersen EM, Nielsen TT, Egeblad H. Serial magnetic resonance imaging of global and regional left ventricular remodeling during 1 year after acute myocardial infarction. Cardiology. 2001;96:106–114. doi: 10.1159/000049092. [DOI] [PubMed] [Google Scholar]
- 34.D'Amario D, Cabral-Da-Silva MC, Zheng H, Fiorini C, Goichberg P, Steadman E, Ferreira-Martins J, Sanada F, Piccoli M, Cappetta D, D'Alessandro DA, Michler RE, Hosoda T, Anastasia L, Rota M, Leri A, Anversa P, Kajstura J. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circulation research. 2011;108:1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, Assmus B, Dimmeler S, Zeiher AM, Hamm C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute st-segment elevation myocardial infarction: Results of the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction study (repair-ami) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–547. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.