Abstract
Objective
To identify potential relationships between plasma 25OHD, C-reactive protein (CRP), coronary artery atherosclerosis (CAA), and coronary artery remodeling in monkeys consuming atherogenic diets.
Methods
Female cynomolgus monkeys (n=74) were fed a casein-lactalbumin (C/L) based, moderately atherogenic diet for 12 months. They then consumed either a soy (n=35) or C/L (n=39) based diet for 32 months. CRP concentrations were then determined and monkeys underwent surgical menopause. Each diet group was then re-randomized to receive soy (n=36) or C/L (n=38). After 32 post-menopausal months, 25OHD, CRP, CAA, and coronary artery remodeling were determined. All monkeys received a women’s equivalent of 1,000 IU/day of 25OHD3 and 1,200 mg/day of calcium, throughout the study.
Results
The pre and post-menopausal dietary protein sources had no effect on post-menopausal 25OHD3 concentrations (p=0.6). Across treatment groups, there was a statistically significant inverse relationship between 25OHD3 concentrations and CRP at necropsy (r=-0.35, p=0.003). A significant inverse correlation between 25OHD3 concentration and the change in CRP, from pre-menopause to post-menopause, was observed (r=-0.32, p=0.007). The significant associations identified between plasma 25OHD3 and CRP remained after controlling for postmenopausal diet. Those monkeys with a greater increase in CRP also had significantly more CAA and less ability to maintain normal lumens by remodeling.
Conclusions
Higher plasma concentrations of 25OHD3 were associated with lower CRP. Lower CRP was associated with less coronary atherosclerosis and improved coronary artery remodeling. These findings suggest that 25OHD3 concentrations are associated with an anti-inflammatory state and may support an association between oral 25OHD3 and cardioprotection.
Keywords: Plasma 25OHD3, C - reactive protein, Cardioprotection, Coronary heart disease, Menopause, coronary artery remodeling
Introduction
It has been estimated that approximately one-third of forty year old women in the United States will develop coronary heart disease (CHD)1. Despite recent declines, CHD remains the number one cause of mortality in woman ≥ 65 years of age2,3. Furthermore, approximately 64% of women who die of CHD had no prior symptoms3. Primary risk factors for CHD in women, as determined by the National Cholesterol Education Program (NCEP), the American Heart Association (AHA), and the American College of Cardiology (ACC), include a personal history of CHD, age over 55, dyslipidemia, family history of premature CHD, diabetes mellitus, smoking, hypertension, obesity, physical inactivity, the metabolic syndrome, and a personal history of peripheral arterial disease2-5. However, the aforementioned risk factors may still underestimate risk in early postmenopausal women. Among young (~55 years of age), non-diabetic postmenopausal women classified as low-risk and requiring no further intervention according to their Framingham risk score, approximately 33% have detectable coronary artery calcium indicative of subclinical atherosclerosis6. Consequently, additional markers have been evaluated to determine their usefulness as indicators of CHD risk. For example, the inflammatory risk marker C-reactive protein (CRP) has been found to be an independent predictor of future cardiovascular events7.
In recent years, plasma 25OHD has become a topic of major interest in the research community. Although the relationship between 25OHD and bone health is well studied8, the current understanding of 25OHD and its direct effect on other physiologic systems is limited. Accordingly, the Institute of Medicine recently reaffirmed the need to advance our knowledge related to 25OHD deficiency and its association with the non-skeletal parameters8. Additionally, it has been well established that inflammation plays an important role in the pathogenesis of cardiovascular disease7,9. The assessment of inflammatory markers, therefore, has been proposed as an approach to improve risk prediction of cardiovascular events. Among the biomarkers of inflammation, CRP appears to have the strongest independent ability to predict cardiovascular events9. More than a dozen prospective epidemiological studies have demonstrated that a single, non-fasting measure of CRP is a strong predictor of future cardiovascular events among individuals without a prior history of cardiovascular disease7.
Recent studies have suggested there may be a relationship between plasma 25OHD concentrations and CHD risk10,11. A higher incidence of CHD and hypertension in higher latitudes, with less sunlight, has been identified in the results of previous studies. For instance, those with a lower exposure to ultraviolet light, and hence a lower vitamin D concentration, may have a higher risk of heart disease, CHD, myocardial infarction, and hypertension12-15. An association between 25OHD and CHD, therefore, could be particularly relevant to elderly women who are at risk for 25OHD deficiency due to an age-associated decline in skin photoisomerization of 7-dehydrocholesterol16 and decreased dietary intake of 25OHD3. Additional risk factors for 25OHD deficiency include, living further from the equator, impaired hydroxylation, impaired renal function, and end organ insensitivity17-22.
The majority of studies investigating the association between plasma 25OHD concentrations and CHD in human subjects are retrospective observational studies. The study presented here utilizes a cross sectional study design of cynomolgus monkeys in which oral 25OHD3 intake, sun exposure, and activity level are controlled, while atherosclerosis extent and arterial remodeling are measured directly at the tissue level. The objective of this study, therefore, was to determine the relationships between 25OHD3, CRP, coronary artery atherosclerosis, and coronary artery remodeling.
Methods
Animals and Diets
Adult female cynomolgus monkeys (Macaca fascicularis; n = 74) were used for this study. The monkeys were imported from Indonesia (Institute Pertanian Bogor), adult status was confirmed, and ages were estimated by epiphyseal closure and dentition. Upon arrival, monkeys underwent a 12 month pre-experimental period, during which they received a controlled casein-lactalbumin (C/L) atherogenic diet. After this period, monkeys were randomized to a diet containing as its main protein source either soy or C/L. Both diets contained identical amounts of fat, carbohydrate and protein per calorie of diet. The diets were equivalent in 25OHD3 and calcium (women’s equivalent 1,000 IU/day and 1,200 mg, respectively). After consuming the diet for an additional 32 months, the monkeys underwent ovariectomy to induce surgical menopause. At this time the monkeys were re-randomized to soy or C/L such that the pre and post menopausal dietary combinations included soy-soy, soy-C/L, C/L-soy, or C/L-C/L. Monkeys consumed the diets for an additional 32 months post-ovariectomy. Body composition measurements were obtained at ovariectomy and at necropsy. An adiposity index for the monkeys was derived by measuring body weight by trunk length, and has a normal range of 35 to 55 kg/m2. Trunk length is measured from the sternal notch to the pubic symphysis.
All procedures involving animals in this study were conducted in compliance with the state and federal laws, standards of the US Department of Health and Human Services (DHHD), and guidelines established by the Wake Forest University Institutional Animal Care and Use Committee (IAcuC) where the animals were housed.
C-reactive Protein Measurement
CRP was measured prior to and 32 months post ovariectomy. Serum CRP was determined by ELISA using a human-based assay from ALPCO Diagnostics (Salem NH). Intra-assay and interassay coefficients of variation were less than 10%.
25OHD3 Measurement
Plasma 25OHD3 concentrations were measured in all 74 female cynomolgus monkeys 32 months post ovariectomy. All animals were housed indoors during the entire length of the study, minimizing any effect of sun exposure. All 25OHD assays were done at The Reading Hospital and Medical Center. Frozen samples (500 ml at -70°C) were transported to The Reading Hospital and Medical Center and protected from direct sunlight as per previously recommended techniques for yielding stable results23. The samples had never been thawed prior to the 25OHD assessments and HPLC - tandem mass spectrometry was used for the 25OHD assays (both 25OHD2 and 25OHD3). Our approach utilized the Shimadzu liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/2) technology. The liquid chromatography-mass spectrometry prepares the sample to be ionized, through physical separation capabilities of liquid chromatography, for mass analysis by injection into the AB Siex 3200 Q Trap mass spectrometer. Vitamin D was provided in the diet as 25OHD3, so all analyses were done with plasma concentrations of 25OHD3.
Coronary Artery Atherosclerosis
At necropsy, the coronary arteries were perfusion fixed with 10% neutral buffered formalin at a pressure of 100 mm Hg. The left anterior descending (LAD), left circumflex (LCX), and right (RCA) coronary arteries were evaluated histomorphometrically to determine both plaque thickness (maximal intimal thickness [MXIT]) and plaque area (intimal area [IA]) as described previously24,25. The mean MXIT and IA for each of the three coronary arteries was then determined and the outcome variable for each was reported and analyzed, independently, as coronary artery atherosclerosis.
Coronary Artery Remodeling
After determining the degree of coronary artery atherosclerosis, as described above, the degree of coronary artery remodeling was assessed. The remodeling process, in which luminal area increases as arterial plaque size increases, has been described previously for both human subjects and macaques24. In this study, remodeling was assessed by comparing the coronary artery plaque size to the luminal area. The degree of coronary artery remodeling was assessed by comparing the plaque size to artery size ratio, plaque size to luminal area ratio, and luminal area to artery size in the three coronary arteries.
Relationships among 25OHD3, CRP and Atherosclerosis
Relationships between CRP changes and coronary artery atherosclerosis among all three coronary arteries were determined. The relationships between 25OHD3 and CRP at necropsy, as well as the relationship between 25OHD3 and the percent change, and absolute change, in CRP during the postmenopausal phase, were investigated. We analyzed those with high (≥ the mean) and low (< the mean) plasma concentrations of 25OHD3 along with high (≥ the mean) and low (< the mean) plasma CRP concentrations. Using these parameters, we compared those with high 25OHD3 / low CRP, high 25OHD3 / high CRP, low 25OHD3 / low CRP, and low 25OHD3 / high CRP. Also, hypothesizing that those with high 25OHD3 / low CRP would have the lowest degree of coronary artery atherosclerosis, we studied this group compared with all the others combined.
Statistical analysis
Body weight data are presented as means and standard deviations. 25OHD3 concentrations were assessed for normality of distribution and were treated as continuous variables. Subjects were dichotomized into high and low 25OHD3 concentration groups based on the determined mean concentration of 25OHD3. Each parameter of interest was compared between two 25OHD3 concentration groups either by student’s t-test or Mann-Whitney test depending upon whether or not data was normally distributed. For assessment of correlation between demographic variables and 25OHD3 concentrations of the entire cohort (n=74), Pearson’s correlation was used for normally distributed continuous variables, and Spearman’s ranked correlation for non-normally distributed variables. Correlations between CRP and 25OHD3 concentrations (considered as both categorical [high vs. low] and continuous variables) were analyzed using Spearman’s ranked correlation. Multiple linear regression analysis was employed to assess predictive relationships between 25OHD3 concentrations and each parameter of interest. All comparisons were done at both the end of menopause (at necropsy) and for the postmenopausal period (ovariectomy to necropsy). SAS 9.2 was used for all analyses, a value of p<0.05 was used to determine a statistical significance.
During construction of figure 3, comparing CRP and coronary artery atherosclerosis as a continuous variable, there was one atherosclerosis data point that was noted to be an outlier value for the intimal area parameters. The data point was more than 10 times higher than the other values. While this value was determined to be a true value, the inclusion in the figure made the graphic representation difficult. While the value was included in the results and data analysis, this subject was excluded from figure 3 in order to eliminate a disproportionate scale. Examination of analytic results was performed, however, and the p value remained unchanged after elimination of this outlier value.
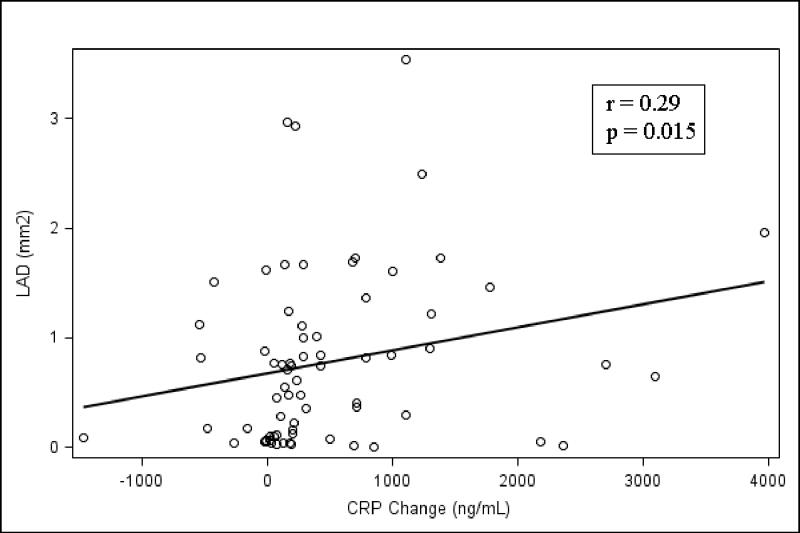
Figure 3.
A correlation between the plaque area of the LAD and the absolute increase in CRP during the 32 month postmenopausal period is shown. A direct correlation between a greater absolute increase in CRP during the menopausal years and significantly greater amounts of coronary artery atherosclerosis, as measured by coronary artery plaque intimal area, was observed (LAD, r = 0.29, p=0.015). LAD = left anterior descending coronary artery; CRP = C-reactive protein.
Results
Of the 74 monkey’s, thirty nine consumed the C/L diet and thirty five consumed the Soy diet during the premenopausal phase (baseline to ovariectomy). During the postmenopausal phase (ovariectomy to necropsy), thirty eight consumed the C/L diet and thirty six consumed the Soy diet. At the end of the postmenopausal life phase, plasma 25OHD3 concentrations ranged from 1.4 to 102 with a mean of 48.9 +/- 16.6 ng/mL. The estimated mean age was 21.5 +/- 3.2 years, the mean weight was 3.4 +/- 0.7 kg and the mean adiposity index was 47.6 +/- 8.7 kg/m2. See table 1 for additional demographic information.
Table 1.
Demographics of four Dietary Groups
| Diet | Soy-Soy (n = 17) | Soy-C/L (n = 18) | C/L-Soy (n = 19) | C/L-C/L (n = 20) | Total (n=74) | P value* |
|---|---|---|---|---|---|---|
| Variables (Mean +/- SD) | ||||||
| Age | 21.4+/-3.0 | 21.3+/-3.6 | 21.8+/-3.1 | 21.3+/-3.1 | 21.5 +/- 3.2 | 0.961 |
| Body weight (at ovariectomy) (kg) | 3.4 +/- 0.7 | 3.5+/- 0.9 | 3.4+/- 0.4 | 3.4+/- 0.7 | 3.4 +/- 0.7 | 0.929 |
| AI (at ovariectomy) | 45.7+/-9.2 | 46.5+/-10.6 | 49.4+/-6.5 | 48.3+/-8.6 | 47.6 +/- 8.7 | 0.568 |
| 25OHD3 Concentration (ng/mL) | 49.4+/-12.1 | 44.4+/-14.0 | 50.2+/-16.4 | 51.5+/- 22.2 | 48.9+/-16.6 | 0.599 |
C/L = casein-lactalbumin
SD = standard deviation
AI = Adiposity Index
Baseline comparison of each parameter of interest between four diet groups was conducted by One-way Analysis of Variance (ANOVA)
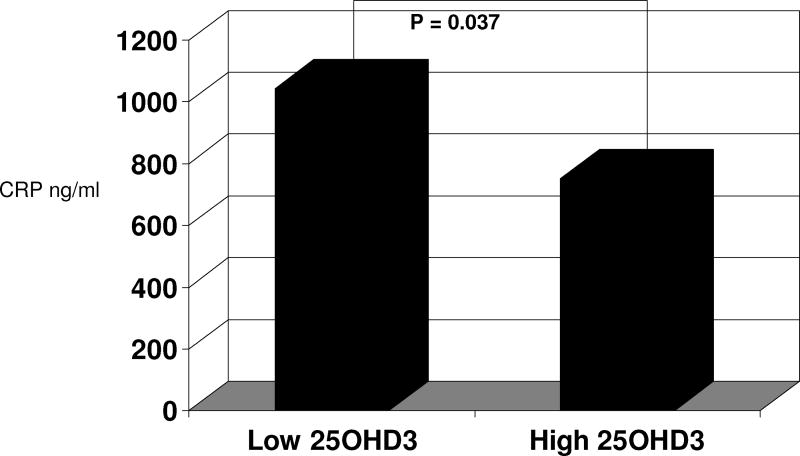
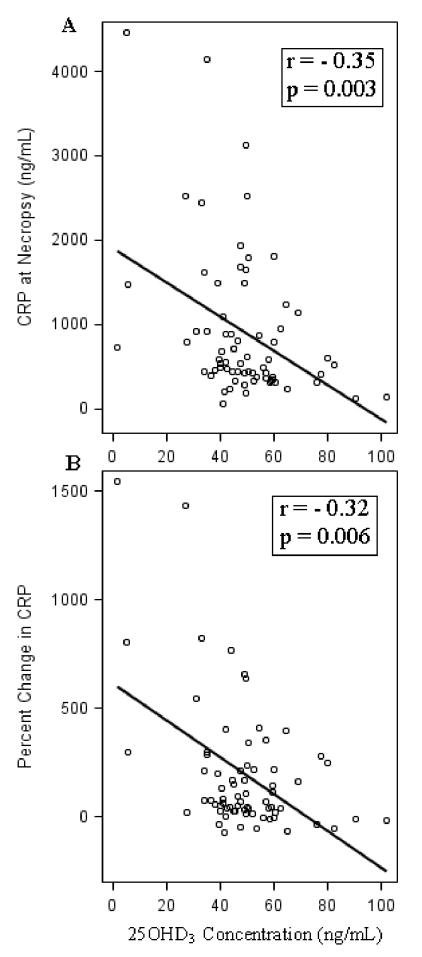
After being postmenopausal for 32 months, monkeys with a higher plasma 25OHD3 concentration (i.e. ≥ the mean) had significantly lower CRP concentrations (750.6 ng/ml +/- 698.1 vs. 1042.3 ng/ml +/- 994.4, respectively, p = 0.04; Effect size Cohen’s d = -0.36; figure 1) than the group with low 25OHD3. Similarly, the continuous data also show a significant inverse relationship between plasma 25OHD3 and serum CRP at necropsy (p = 0.003, r = -0.35), figure 2A. When controlling for the postmenopausal diet (soy versus C/L) in a multiple linear regression analysis, this relationship was unchanged (p = 0.0004, Adjusted R-Square = 0.15). The relationship between the percent change in CRP from the end of the premenopausal period until the end of the postmenopausal time period (32 months) and 25OHD3 concentrations was also inverse (r=-0.32, p = 0.006), figure 2B. When controlling for the postmenopausal diet (soy vs. C/L) in a multiple linear regression analysis, the relationship was again unchanged (p < 0.0001, Adjusted R-Square = 0.20). Similarly, as the plasma concentration of 25OHD3 increased, the absolute increase in CRP was significantly lower (p = 0.007, r = -0.31). Again, when we controlled for the postmenopausal diet (soy vs. C/L) in a multiple linear regression analysis, this relationship was still significant (p = 0.0006, Adjusted R-Square = 0.19).
Figure 1.
C-reactive protein (ng/mL) values in postmenopausal monkeys with lower vs. higher plasma 25OHD3 concentrations (< 48.9 ng/mL vs. ≥ 48.9 ng/mL, respectively).
Figure 2.
Relationship between 25OHD3 concentration and C-reactive protein (CRP) concentration (ng/mL) at necropsy (A) and with percent change in C-reactive protein (CRP) concentration (ng/mL) from ovariectomy to necropsy (32 months) (B) in cynomolgus monkeys (Macaca fascicularis).
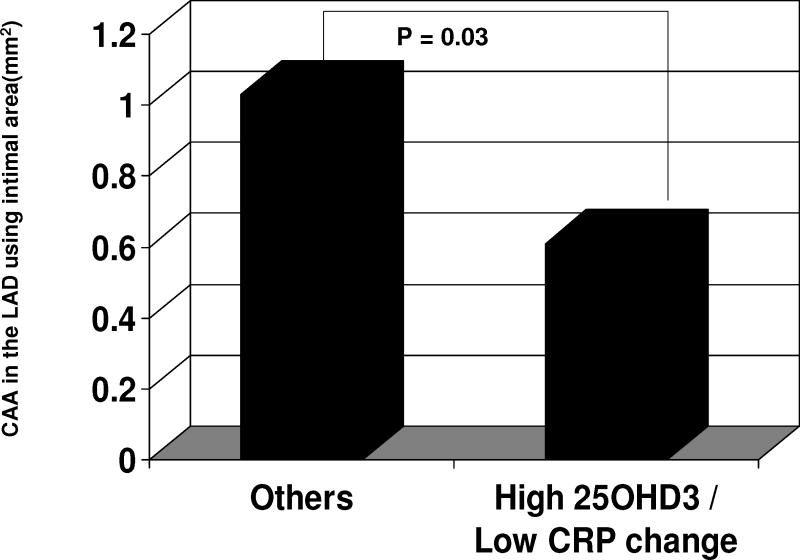
As the absolute CRP increased, atherosclerotic plaque area (IA) and maximal intimal thickness (MXIT) were increased in all three main coronary arteries (LAD, LCX, and RCA), Figure 3. LAD atherosclerosis extent was decreased in monkeys with high plasma 25OHD3 concentrations (in the upper half of the mean) and low CRP change (in the lower half of the mean) compared to all other monkeys (those without high 25OHD3 and low CRP change), p=0.03, Cohen’s d =0.50, figure 4. This relationship was also observed with plaque size (MXIT) in the RCA (p=0.04, Cohen’s d =0.58), but not in the LAD or LCX.
Figure 4.
Left anterior descending (LAD) coronary artery atherosclerosis (mm2), in postmenopausal monkeys with high 25OHD3 / low C-reactive protein change (ng/mL) compared to all the others. CRP change = Absolute change in C-reactive protein concentration during the menopause years; CAA = coronary artery atherosclerosis.
We also observed the usual association between the ratio of mean lumen area to mean intimal area (plaque size) of the LCX, LAD, and RCA. As coronary artery plaque size (IA) increased there was a significant association with increases in the lumen area (LA) of all three main coronary arteries (LCX, r = 0.57, p<0.0001; LAD, r = 0.55, p<0.0001; RCA, r = 0.25, p=0.0355), indicative of artery remodeling. However, remodeling appeared to be compromised in monkeys that experienced an increase in CRP concentration during the menopausal years. CRP was positively associated with the intimal area:artery size ratios of the LCX, LAD, and RCA. This finding represents a consistent finding, in all three main coronary arteries, of a correlation between a greater absolute increase in CRP during the menopausal years and a significant compromise in coronary artery remodeling (LCX, r = 0.32, p=0.007; LAD, r = 0.32, p=0.007; RCA, r = 0.33, p=0.005). Finally, we showed that the absolute change in CRP was inversely related to the luminal area:artery size ratio for all three main coronary arteries (LAD, LCX, and RCA). This again confirmed an enhanced coronary artery remodeling with a lower CRP increase during menopause (figure 5).
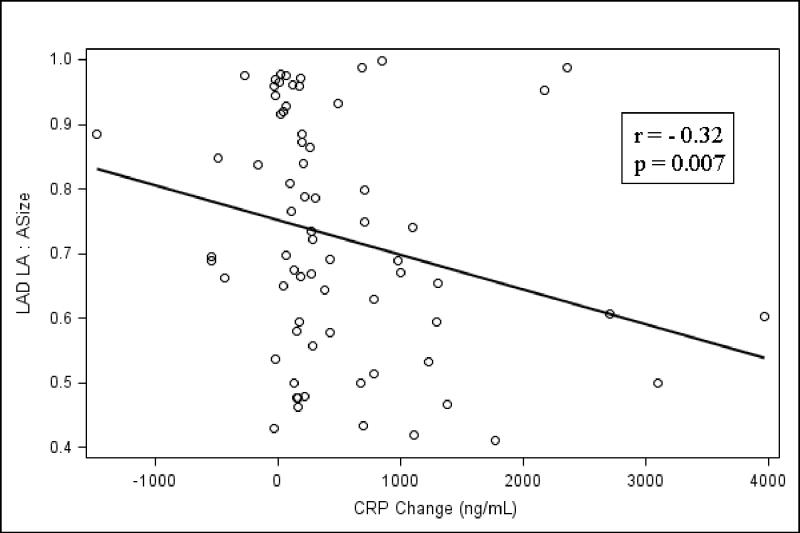
Figure 5.
This figure demonstrates a correlation between the luminal area:artery size ratio of the LAD vs. the absolute increase in CRP during the menopausal years. This represents a direct correlation between a greater absolute increase in CRP during the menopausal years and a significantly greater decrease in luminal area: artery size ratio, representing a compromise in coronary artery remodeling (LAD, r = -0.32, p=0.007). LAD = left anterior descending coronary artery; ASize = Artery size; LA = Luminal area; CRP = C-reactive protein.
Discussion
The results of our study demonstrated an inverse relationship between plasma concentrations of 25OHD3 and CRP. The association was apparent when considering 25OHD3 both as a continuous variable and when comparing higher versus lower 25OHD3 plasma concentrations. We also demonstrated that higher CRP concentrations, as well as increasing CRP concentrations during the menopause, were associated with greater coronary atherosclerosis.
What does the relationship between 25OHD3 and CRP identified in our study mean? Certainly CRP is an acute phase reactant and as such has been shown to rise dramatically during inflammatory processes occurring in the human body26,27. Current research has demonstrated that these markers play significant roles in a variety of acute and chronic diseases including infections, systemic inflammatory processes, tissue injury, hypertension, the metabolic syndrome, and most notably CHD26,27. 25OHD3 has been recognized as an immune-modulator that may suppress pro-inflammatory cytokine expression and regulate immune cell activity28,29. Additionally, it is believed that 25OHD3 supplementation may be associated with a reduction in pro-inflammatory cytokines in patients with heart failure30. It is important to note that this study was not designed to assess the effects of differential Vitamin D supplementation, as all animals received equivalent levels of 25OHD3 in their diet. Individual variation in circulating 25OHD3 is likely due, in part, to genetic determinants and responses to atherogenic diets, which may influence absorption and degradation in the liver, kidney, and elsewhere. Since CRP is produced by the liver, it is possible the inverse relationship between 25OHD3 and CRP is related to hepatic function, and that hepatic inflammation causes increased 25OHD3 turnover. More work needs to be done to explore these potential mechanisms. While there is some evidence to suggest 25OHD deficiency may be associated with higher rates of cardiovascular disease10,11,31, well designed prospective data to support this belief are lacking8. Additionally, at least one recent randomized study did not show that 25OHD3 supplementation changed circulating cytokine levels among healthy adults32.
Some of the best data available, in fact, has also not been consistent with a cardio-protective role in regard to 25OHD. For example, data from the Women’s Health Initiative, analyzing 36,282 postmenopausal women randomized to calcium and 25OHD3 supplementation, was recently published33. This study suggested that calcium and 25OHD3 supplementation was not associated with a risk of coronary or cerebrovascular events in generally healthy postmenopausal women over a 7-year period. Due to limitations of the study, however, it is still premature to draw definitive conclusions based on these findings. While these data are from a prospective trial, it was originally designed to evaluate the effects of calcium and 25OHD3 supplementation on fracture, not cardiovascular disease. Also, the dose of 25OHD3 (400 IU daily) might be inadequate and insufficient to achieve optimal plasma 25OHD3 concentrations33,34. In addition, the proportion of patients with coexisting hypertension at baseline was significantly higher in the 25OHD3 supplementation group. Since clinical evidence of 25OHD as a protective factor for cardiovascular disease in postmenopausal women is limited, additional data on potential mechanisms for cardioprotection are necessary. The inverse relationship between plasma 25OHD and CRP observed here suggests either an anti-inflammatory, and perhaps cardioprotective, effect of Vitamin D or a potential explanation, i.e. systemic inflammation, for a low 25OHD3 concentration in those with cardiovascular disease.
While a direct causal relationship between elevated CRP and coronary artery atherosclerosis in human subjects has not been proven, CRP is nevertheless frequently used in CHD risk assessment algorithms9,35-37. Not shown previously, for either human or nonhuman primates, is an association between coronary artery remodeling and plasma CRP concentrations. Despite the difficulty proving this in human cohorts, we were able to document that the increase in CRP over time, and during the menopausal years, correlated with extent of coronary artery atherosclerosis. We found that those with the highest plasma 25OHD3 concentrations and the lowest increase of CRP over time, as expected, were afforded the greatest protection against coronary artery atherosclerosis. We also determined that those with the smallest increases in CRP during the menopausal period had the greatest degree of coronary artery remodeling, a finding consistent with decreased risk for CHD and cardiovascular events24. Clearly we have shown strong evidence that lower plasma concentrations of 25OHD3 are associated with higher systemic inflammation, as measured by CRP, and this in turn is associated with greater coronary artery atherosclerosis and worsening coronary artery remodeling. 25OHD3, therefore, could play a cardio-protective role evidenced by the reduction of this marker, or may at least indicate a less atherogenic milieu. In addition to identifying and correlating a direct association between 25OHD3 and coronary artery atherosclerosis, the correlation with other disease entities, as listed above, could also prove beneficial through future prospective trials.
The limitations to this study include the following. While this cynomolgus monkey model has proven to be effective in studying menopause related chronic diseases, particularly cardiovascular disease38, the relationships identified in this study may not be the same as those in human beings, hence further studies in human cohorts are needed. The small sample size in our monkey cohort may have contributed to a type 2 error, limiting the identification of further significant relationships. While it is possible that the various dietary regimens could have affected the inflammatory markers, we demonstrated that the source of dietary protein had no effect on the plasma concentrations of 25OHD3. In addition, regression analyses controlling for the diets revealed that the dietary changes did not alter the significant associations between 25OHD3 and CRP. Because we do not definitively know the change in 25OHD3 concentration over the course of the study, we cannot conclusively determine whether inflammation leads to a reduced 25OHD3 or whether a higher 25OHD3 leads to reduced inflammation. We believe it is highly unlikely, however, that the plasma 25OHD3 concentrations in these monkeys varied significantly over time because of the strict control over their dietary 25OHD3 and their environment, both of which remained constant during the experiment. In addition, because genetic factors are likely responsible for at least some of the observed variations in 25OHD3 concentrations, it is plausible that these same factors may be influencing CRP concentrations. Therefore, despite our data showing a clear association between 25OHD3 and CRP, further prospective data will be needed to clarify this relationship along with the temporal association.
Advantages of the non-human primate model include the ability to control for many of the confounding variables in human studies, including diet, 25OHD3 intake, sun exposure, and general compliance. Additional strengths include the elimination of various confounding factors existing in human studies such as coexisting medical conditions (i.e. type II diabetes) which has been linked to elevations in pro-inflammatory markers, especially CRP28 and concurrent hormonal therapy which has been associated with increased CRP concentrations39. Other confounding factors include exogenous 25OHD3/calcium or other supplementation (known or unknown) along with exercise, which has been associated with decreased inflammatory markers including CRP40. For these reasons, observed findings in this model may yield results that are less biased than those in human cohorts. It will be beneficial, therefore, to examine these findings in the general postmenopausal population.
Conclusion
We have identified a significant inverse correlation between 25OHD3 and the inflammatory marker CRP. This increase in CRP, likewise, was correlated with a greater degree of coronary artery atherosclerosis and inhibited coronary artery remodeling. This association was strong and independent of any of the menopausal dietary changes. These findings suggest that circulating 25OHD3 concentrations are associated with an anti-inflammatory state. These findings support previous suggestions that higher 25OHD3 concentrations may result in cardiovascular protection. Further investigations are required to determine the mechanism behind these relationships.
Acknowledgments
The funding sources for this research and manuscript preparation were the research budgets of the Wake Forest University Primate Center and The Reading Hospital and Medical Center. In addition, the original study was supported by the following grants from the National Institutes of Health: HL079421 (JRK), PPG HL 45666 (TBC, JRK), and AG027847 (SEA).
Footnotes
These data were presented in abstract form September 22nd, 2011 at the NAMS 22nd annual Meeting in Washington, DC. These data and results, however, have not been published in manuscript form and have not been submitted previously to another journal.
Conflict of Interest: Thomas B. Clarkson, DVM is a member of an advisory committee to Pfizer pharmaceuticals and has been supported with a research grant from Pfizer. He is also the recipient of a research grant from Merck. The other authors have no conflicts of interest.
References
- 1.Lloyd-Jones DM, Larson MG, Beiser A, Levey D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. AHA Statistical Update: Heart Disease and Stroke Statistics—2011 Update; A Report From the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women 2011 Update: A Guideline From the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 5.Mosca L, Grundy SM, Judelson D, et al. AHA/ACC scientific statement: consensus panel statement. Guide to preventative cardiology for women. American Heart Association/American College of Cardiology. J Am Coll Cardiol. 1999;33:1751–5. doi: 10.1016/s0735-1097(99)00190-4. [DOI] [PubMed] [Google Scholar]
- 6.Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, et al. Framingham Risk Equation Underestimates Subclinical Atherosclerosis Risk in Asymptomatic Women. Atherosclerosis. 2006;184:201–6. doi: 10.1016/j.atherosclerosis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. [February 2, 2012];Brief Report, November 2010. Dietary Reference Intakes for Calcium and Vitamin D. Available at: http://www.iom.edu/~/media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20Calcium%202010%20Report%20Brief.pdf.
- 9.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnatz PF, Nudy M, O’Sullivan DM, Ethun K, Appt SE, Clarkson TB. Identification of a Mechanism for Increased Cardiovascular Risk among Individuals with Low Vitamin D Concentrations. Menopause. 2011;18(9):994–1000. doi: 10.1097/gme.0b013e318212539d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. Q J Med. 1996;89:579–89. doi: 10.1093/qjmed/89.8.579. [DOI] [PubMed] [Google Scholar]
- 13.Rostand SG. Untraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman JP, Giovannucci E, Homes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 16.Tsai KS, Wahner HW, Offor KP, et al. Effect of aging vitamin D stores and bone density in women. Calcif Tissue Int. 1987;40:241–3. doi: 10.1007/BF02555255. [DOI] [PubMed] [Google Scholar]
- 17.Binkley N, Novotny R, Krueger D, et al. Low vitamin d status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 18.Taskapan H, Ersoy FF, Passadakis PS, Tam P, Memmos DE, et al. Severe vitamin D deficiency in chronic renal failure patients on peritoneal dialysis. Clin Nephrol. 2006;66(4):247–55. doi: 10.5414/cnp66247. [DOI] [PubMed] [Google Scholar]
- 19.Compston JE. Hepatic osteodystrophy: vitamin D metabolism in patients with liver disease. Gut. 1986;27:1073–90. doi: 10.1136/gut.27.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dibble JB, Sheridan P, Losowsky MS. A survey of vitamin D deficiency in gastrointestinal and liver disorders. Quarterly Journal of Medicine. 1984;53:119–134. [PubMed] [Google Scholar]
- 21.Hewison M, Rut AR, Kristjansson K, et al. Tissue resistance to 1,25-dihydroxyvitamin D without a mutation of the vitamin D receptor gene. Clin Endocrinol. 1003;39:663–70. doi: 10.1111/j.1365-2265.1993.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 22.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JG, Elder PA. Serum 25-OH vitamin D2 and D3 are stable under exaggerated conditions. Clin Chem. 2008;54(11):1931–2. doi: 10.1373/clinchem.2008.111526. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson TB, Prichard RW, Morgan TM, Petrick GS, Klein KP. Remodeling of Coronary Arteries in Human and Nonhuman Primates. JAMA. 1994;271(4):289–94. [PubMed] [Google Scholar]
- 25.Clarkson TB, Alexander NJ, Morgan TM. Atherosclerosis of cynomolgus monkeys hyper- and hyporesponsive to dietary cholesterol. Lack of effect of vasectomy. Arterioscler Thromb Vasc Biol. 1988;8:488–98. doi: 10.1161/01.atv.8.5.488. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhardt SU, Thiele JR, Bannasch H, Stark GB, Peter K. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. 2009 Dec;8(23):3885–92. doi: 10.4161/cc.8.23.10068. [DOI] [PubMed] [Google Scholar]
- 27.Miura T. C-Reactive Protein (CRP) Is Not a Mere Marker, but an Active Pathogenic Substance. Circ J. 2011 Jun 24;75(7):1579–80. doi: 10.1253/circj.cj-11-0528. Epub 2011 Jun 3. [DOI] [PubMed] [Google Scholar]
- 28.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile, 1,25- Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Research and Clinical Practice. 2007;77:47–57. doi: 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Mathieu C, Van Etten E, Decallonne B, Giulietti A, Gysemans C, Bouillon R, et al. Vitamin D and 1,25-dihydroxyvitamin D3 as modulators in the immune system. J Steroid Biochem Mol Biol. 2004;89(90):449–52. doi: 10.1016/j.jsbmb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168(11):1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin D and serum cytokines in a randomized clinical trial. International Journal of Endocrinology. 2010:7. doi: 10.1155/2010/305054. Article ID 305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M, et al. Calcium/Vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–54. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 34.Vitamin D. Research appears to support greater daily intake. [February 2, 2012];Endocrine today. From cancer to stroke to autoimmune diseases, research is piling up on vitamin D’s potential effects. Available at: http://www.endocrinetoday.com/view.aspx?rid=35928#jump Posted on January 10, 2009.
- 35.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 36.Dursunoglu D, Goksoy H, Ozturk M, Rota S. A relationship between CRP, adiponectin and Gensini score in the patients with coronary artery disease. Anadolu Kardiyol Derg. 2011;11(3):195–200. doi: 10.5152/akd.2011.052. [DOI] [PubMed] [Google Scholar]
- 37.Soysal D, Karakus V, Yavas HH, Biceroglu S, Koseoglu M, Yesil M. C-reactive protein in unstable angina pectoris and its relation to coronary angiographic severity and diffusion scores of coronary lesions. Anadolu Kardiyol Derg. 2010;10(5):421–8. doi: 10.5152/akd.2010.140. [DOI] [PubMed] [Google Scholar]
- 38.Clarkson TB, Mehaffey MH. Coronary heart disease of females: lessons learned from nonhuman primates. Am J of Primatol. 2009;71:785–93. doi: 10.1002/ajp.20693. [DOI] [PubMed] [Google Scholar]
- 39.Vongpatanasin W, Tuncel M, Wang Z, Arbique D, Mehrad B, Jialal I. Different effects of oral versus transdermal estrogen replacement therapy on C-reactive protein in postmenopausal women. J Am Coll Cardio. 2003;41:1358–63. doi: 10.1016/s0735-1097(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 40.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. 2004;52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]