Abstract
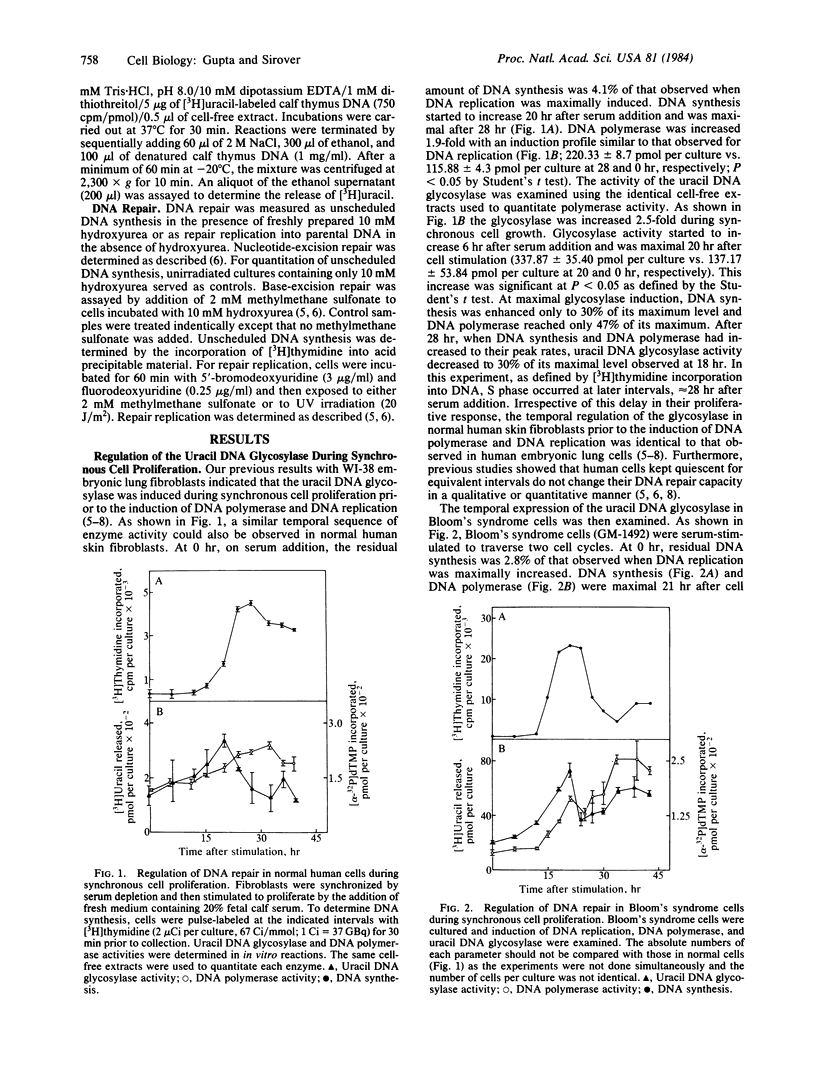
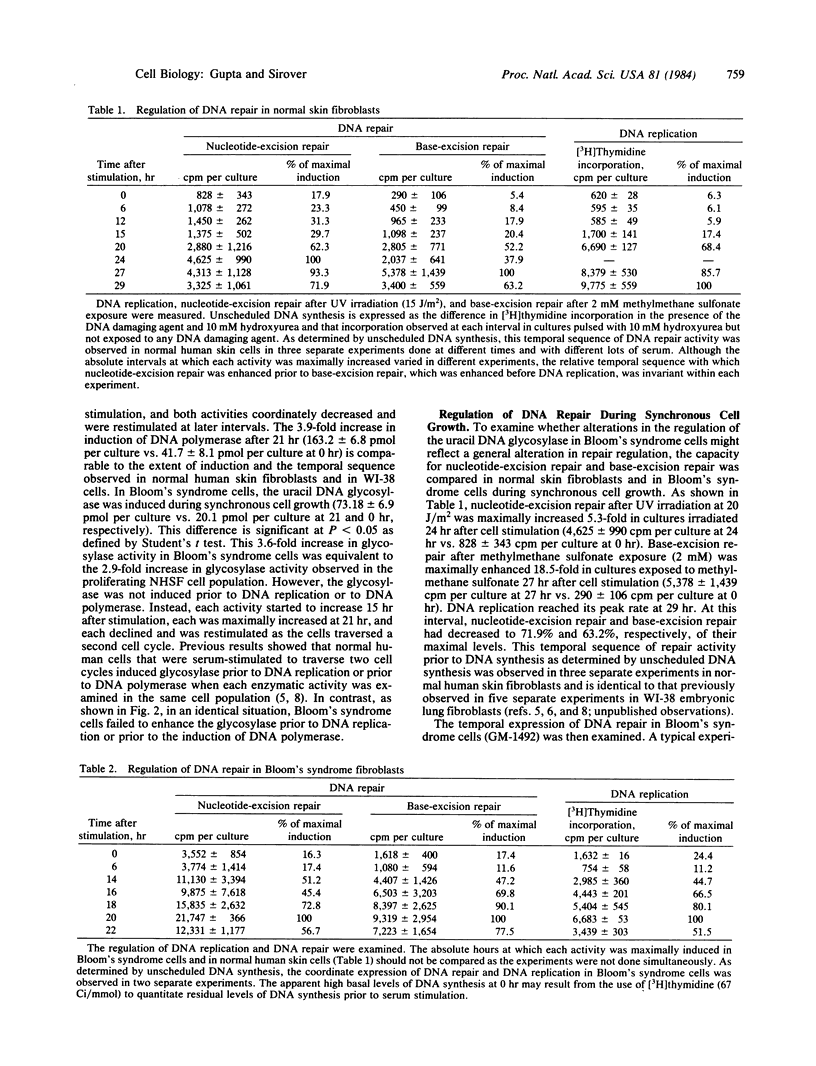
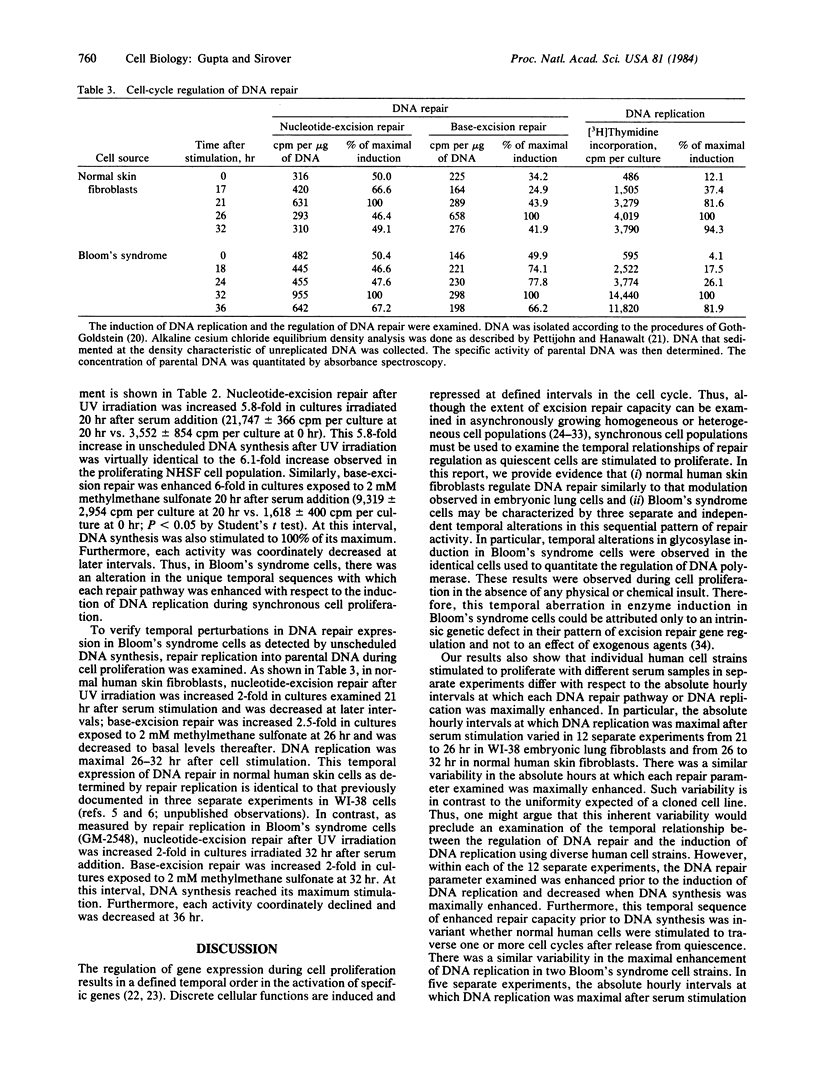
The temporal regulation of DNA repair during synchronous cell proliferation was examined in normal human skin fibroblasts and in Bloom's syndrome skin fibroblasts. Normal human cells regulated DNA repair in a defined temporal sequence prior to the induction of DNA replication. Nucleotide-excision repair was stimulated prior to the induction of base-excision repair, which itself was increased prior to the induction of DNA replication. This temporal sequence was observed (i) by quantitation of the induction of the base-excision repair enzyme uracil DNA glycosylase during cell proliferation in the absence of cellular insult and (ii) by quantitation of nucleotide-excision repair after UV irradiation or base-excision repair after exposure to methylmethane sulfonate. In contrast, Bloom's syndrome cells were characterized by specific alterations in this temporal sequence of gene regulation, such that DNA repair was not enhanced prior to the induction of DNA replication. Nucleotide-excision repair, base-excision repair, and the uracil DNA glycosylase were induced in a temporal sequence identical to that observed for DNA polymerase and for DNA replication. The inability of Bloom's syndrome cells to enhance DNA repair prior to DNA replication suggests that miscoding lesions remain in DNA and are replicated during cell proliferation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprelikova O. N., Tomilin N. V. Activity of uracil-DNA glycosylase in different rat tissues and in regenerating rat liver. FEBS Lett. 1982 Jan 25;137(2):193–195. doi: 10.1016/0014-5793(82)80347-5. [DOI] [PubMed] [Google Scholar]
- Bertazzoni U., Stefanini M., Noy G. P., Giulotto E., Nuzzo F., Falaschi A., Spadari S. Variations of DNA polymerase-alpha and -beta during prolonged stimulation of human lymphocytes. Proc Natl Acad Sci U S A. 1976 Mar;73(3):785–789. doi: 10.1073/pnas.73.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant E. M., Hoehn H., Martin G. M. Normalisation of sister chromatid exchange frequencies in Bloom's syndrome by euploid cell hybridisation. Nature. 1979 Jun 28;279(5716):795–796. doi: 10.1038/279795a0. [DOI] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and its coupling to DNA replication in eukaryotic cells. Biochim Biophys Acta. 1978 Dec 11;516(4):489–516. doi: 10.1016/0304-419x(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Craddock V. M., Henderson A. R. The activity of 3-methyladenine DNA glycosylate in animal tissues in relation to carcinogenesis. Carcinogenesis. 1982;3(7):747–750. doi: 10.1093/carcin/3.7.747. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Gombar C. T., Katz E. J., Magee P. N., Sirover M. A. Induction of the DNA repair enzymes uracil DNA glycosylase and 3-methyladenine DNA glycosylase in regenerating rat liver. Carcinogenesis. 1981;2(7):595–599. doi: 10.1093/carcin/2.7.595. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Repair of DNA damaged by alkylating carcinogens is defective in xeroderma pigmentosum-derived fibroblasts. Nature. 1977 May 5;267(5606):81–82. doi: 10.1038/267081a0. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Cell cycle regulation of DNA repair in normal and repair deficient human cells. Chem Biol Interact. 1981 Jul;36(1):19–31. doi: 10.1016/0009-2797(81)90026-0. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Sequential stimulation of DNA repair and DNA replication in normal human cells. Mutat Res. 1980 Sep;72(2):273–284. doi: 10.1016/0027-5107(80)90042-1. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Stimulation of the nuclear uracil DNA glycosylase in proliferating human fibroblasts. Cancer Res. 1981 Aug;41(8):3133–3136. [PubMed] [Google Scholar]
- Gupta R. S., Goldstein S. Diphtheria toxin resistance in human fibroblast cell strains from normal and cancer-prone individuals. Mutat Res. 1980 Dec;73(2):331–338. doi: 10.1016/0027-5107(80)90198-0. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Shearman C. W., Loeb L. A. Mutagenesis in vitro by depurination of phiX174 dna. Nature. 1981 May 28;291(5813):349–351. doi: 10.1038/291349a0. [DOI] [PubMed] [Google Scholar]
- Lavin M. F., Kidson C. Repair of ionizing radiation induced DNA damage in human lymphocytes. Nucleic Acids Res. 1977 Nov;4(11):4015–4022. doi: 10.1093/nar/4.11.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewensohn R., Killander D., Ringborg U., Lambert B. Increase of uv-induced DNA repair synthesis during blast transformation of human lymphocytes. Exp Cell Res. 1979 Oct 1;123(1):107–110. doi: 10.1016/0014-4827(79)90426-9. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Karlström O. Heat-induced depyrimidination of deoxyribonucleic acid in neutral solution. Biochemistry. 1973 Dec 4;12(25):5151–5154. doi: 10.1021/bi00749a020. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Jr, DiPaolo J. A. Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment. Nature. 1978 Sep 14;275(5676):130–132. doi: 10.1038/275130a0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pedersen R. A., Mangia F. Ultraviolet-light-induced unscheduled DNA synthesis by resting and growing mouse oocytes. Mutat Res. 1978 Mar;49(3):425–429. doi: 10.1016/0027-5107(78)90113-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Perry W., Bennett R. A. Effect of partial hepatectomy on removal of O6-methylguanine from alkylated DNA by rat liver extracts. Biochem J. 1981 Jul 1;197(1):195–201. doi: 10.1042/bj1970195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Stimulation of transfer of methyl groups from O6-methylguanine in DNA to protein by rat liver extracts in response to hepatotoxins. Carcinogenesis. 1981;2(11):1195–1200. doi: 10.1093/carcin/2.11.1195. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L. Regulation of O6-methylguanine-DNA methyltransferase levels in rat liver and kidney. Cancer Res. 1983 Mar;43(3):972–975. [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero D., Norin A., Karran P., Strauss B. DNA excision-repair deficiency of human peripheral blood lymphocytes treated with chemical carcinogens. Cancer Res. 1976 Apr;36(4):1397–1403. [PubMed] [Google Scholar]
- Sirover M. A. Induction of the DNA repair enzyme uracil-DNA glycosylase in stimulated human lymphocytes. Cancer Res. 1979 Jun;39(6 Pt 1):2090–2095. [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Erroneous base-pairing induced by a chemical carcinogen during DNA synthesis. Nature. 1974 Nov 29;252(5482):414–416. doi: 10.1038/252414a0. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Restriction of carcinogen-induced error incorporation during in vitro DNA synthesis. Cancer Res. 1976 Feb;36(2 Pt 1):516–523. [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F., Cholon J. J. An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro. 1973 May-Jun;8(6):466–472. doi: 10.1007/BF02615948. [DOI] [PubMed] [Google Scholar]
- Warren S. T., Schultz R. A., Chang C. C., Wade M. H., Trosko J. E. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]


