Abstract
BACKGROUND AND PURPOSE
Elevating levels of endocannabinoids with inhibitors of fatty acid amide hydrolase (FAAH) is a major focus of pain research, purported to be a safer approach devoid of cannabinoid receptor-mediated side effects. Here, we have determined the effects of sustained pharmacological inhibition of FAAH on inflammatory pain behaviour and if pharmacological inhibition of FAAH was as effective as genetic deletion of FAAH on pain behaviour.
EXPERIMENTAL APPROACH
Effects of pre-treatment with a single dose, versus 4 day repeated dosing with the selective FAAH inhibitor, URB597 (i.p. 0.3 mg·kg−1), on carrageenan-induced inflammatory pain behaviour and spinal pro-inflammatory gene induction were determined in rats. Effects of pain induction and of the drug treatments on levels of arachidonoyl ethanolamide (AEA), palmitoyl ethanolamide (PEA) and oleolyl ethanolamide (OEA) in the spinal cord were determined.
KEY RESULTS
Single, but not repeated, URB597 treatment significantly attenuated the development of inflammatory hyperalgesia (P < 0.001, vs. vehicle-treated animals). Neither mode of URB597 treatment altered levels of AEA, PEA and OEA in the hind paw, or carrageenan-induced paw oedema. Single URB597 treatment produced larger increases in AEA, PEA and OEA in the spinal cord, compared with those after repeated administration. Single and repeated URB597 treatment decreased levels of immunoreactive N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) in the spinal cord and attenuated carrageenan-induced spinal pro-inflammatory gene induction.
CONCLUSION AND IMPLICATIONS
Changes in the endocannabinoid system may contribute to the loss of analgesic effects following repeated administration of low dose URB597 in this model of inflammatory pain.
Keywords: Anandamide, inflammatory pain behaviour, FAAH, oleoyl ethanolamide, palmitoyl ethanolamide, spinal, URB597, AM251, GW6471
Introduction
Cannabinoid-based medicines have analgesic activity, but their side effect profile, due to the global activation of cannabinoid CB1 receptors, limits their utility (Pertwee, 2009; receptor nomenclature follows Alexander et al., 2011). Novel strategies in this field have focused on the modulation of the endocannabinoid signalling system. The two major endocannabinoids are the N-acylethanolamine (NAE) anandamide (AEA) and the ester 2-arachidonoylglycerol (2-AG). AEA and other biologically active endogenous NAEs are synthesized by various enzymic pathways, of which the N-acylphosphatidylethanolamine phospholipase D (NAPE–PLD) pathway is the best characterized (see Wang and Ueda, 2009). These NAEs are predominantly degraded by fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996; see McKinney and Cravatt, 2005; Ahn et al., 2008). Increased neuronal activity is associated with increased synthesis of the endocannabinoids and tissue-specific elevations in the endocannabinoids have been reported in various models of pain (see Sagar et al., 2009).
Pharmacological inhibition of FAAH elevates levels of NAEs and attenuates inflammatory pain responses (Jayamanne et al., 2006; Jhaveri et al., 2008; Ahn et al., 2009; Karbarz et al., 2009; Pertwee, 2009) via the activation of CB1, CB2 (Jayamanne et al., 2006; Naidu et al., 2009) and PPARα receptors (LoVerme et al., 2006; Sagar et al., 2008). We have shown that spinal inhibition of FAAH produces a CB1 receptor-mediated attenuation of noxious-evoked responses of spinal neurones in a model of neuropathic pain (Jhaveri et al., 2006) and joint pain (Sagar et al., 2010). Studies using FAAH knockout mice have provided corroborative evidence that elevation of NAEs attenuates inflammatory pain responses (Cravatt et al., 2004; Chang et al., 2006; Naidu et al., 2010). Significantly, there is no evidence for FAAH inhibition producing abuse potential (Justinova et al., 2008; Adamczyk et al., 2009). Given that inflammatory and chronic pain states are associated with tissue-selective elevations in endocannabinoids per se (see Sagar et al., 2009), understanding the potential changes in the endocannabinoid system under these conditions, and the effect of prolonged pharmacological attenuation of FAAH activity on the endocannabinoid system under basal conditions and in a model of pain, is essential.
The aim of our study was to determine the effects of sustained pharmacological inhibition of FAAH on inflammatory pain behaviour and spinal nociceptive signalling pathways. To this end, we used a relatively short duration model of inflammatory pain (carrageenan model) to ensure that the level of inhibition of FAAH activity was stable over the time course of the model. The carrageenan model of acute inflammatory pain produces a robust hind paw oedema and hyperalgesia, accompanied by the spinal induction of inflammation-associated enzymes, such as COX-2 and microsomal PGE synthase-1 (mPGES-1) (Hay and de Belleroche, 1997; Samad et al., 2001; Guay et al., 2004), and the elevation of spinal pro-inflammatory cytokines (Tao et al., 2003; Song et al., 2009) within 3 h. Systemic administration of URB597 produces an elevation in AEA for up to 6 h (Fegley et al., 2005), ensuring stable elevation of NAEs over the time frame of the model. In this study we report that, despite acute and repeated treatment with the FAAH inhibitor URB597 producing a comparable inhibition of spinal cord FAAH activity and producing significant effects on carrageenan-induced spinal pro-inflammatory gene expression, only acute URB597 treatment attenuated behavioural hyperalgesia. Acute URB597 treatment produced greater elevations of spinal NAEs, compared with repeated treatment, and we demonstrate adaptive changes in elements of the endocannabinoid system in the spinal cord under these conditions. Our data suggest that there are differences in the effects of sustained pharmacological inhibition of FAAH, compared with those after genetic deletion of FAAH (FAAH knockout), on inflammatory pain behaviour, which may have important therapeutic implications.
Methods
All animal care and experimental procedures were carried out in accordance with the UK Home Office Animals (Scientific Procedures) Act (1986) and the guidelines of the Committee for Research and Ethical Issues of IASP. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Experiments were performed on 120 male Sprague–Dawley rats (225–275 g). Animals had free access to food and water and were group-housed during the experiments. To determine whether sustained inhibition of FAAH produced analgesia comparable to that after a single dose of URB597 in a model of acute inflammatory pain, some rats were given URB597 daily before the establishment of the pain model. Rats were treated once daily with URB597 (0.3 mg·kg−1) or vehicle (i.p.) for 3 days before the day of the behavioural studies; these groups are described as repeated URB597 treatment or repeated vehicle treatment. On the fourth day, rats received a final dose of 0.3 mg·kg−1 URB597 or vehicle (i.p.) 30 min before injection of λ-carrageenan (Sigma, Gillinghm, Dorset, UK; 2 mg in 100 µL saline). Just before injection of carrageenan, the ipsilateral and contralateral paw volume was measured with a plethysmometer (average of three readings), and then carrageenan was injected into the plantar surface of the left hind paw under brief isoflurane (3%) inhalation anaesthesia (in a gas mixture: 34% O2; 66% N2O). The rationale for dosing with URB597 for 4 days was based on the earlier report of the effects of 4 day treatment with URB597 on levels of AEA in the brain and neurophysiological endpoints (Gobbi et al., 2005). As intraplantar injection of carrageenan produces a dynamic inflammatory pain response, which varies over time, both in terms of modality and magnitude, pharmacological intervention to produce an acute versus sustained block of FAAH at a comparable stage of the inflammatory pain response was not achievable with treatments given after the carrageenan injection. Separate groups of rats received a single dose of URB597 (0.3 mg·kg−1) or vehicle (i.p.) 30 min prior to intraplantar injection of carrageenan, described as acute URB597 treatment or acute vehicle treatment. Control groups received an intraplantar injection of physiological saline (100 µL) in an identical manner to the injection of carrageenan.
Assessment of hyperalgesia and hind paw volume
The following groups were used for the first study: repeated URB597 treatment plus hind paw carrageenan (n= 9); repeated vehicle treatment plus hind paw carrageenan (n= 8); acute URB597 plus hind paw carrageenan (n= 10), acute vehicle plus hind paw carrageenan (n= 8), repeated URB597 treatment plus hind paw saline (n= 6); repeated vehicle treatment plus hind paw saline (n= 6); acute URB597 plus hind paw saline (n= 5), acute vehicle plus hind paw saline (n= 6). Hyperalgesia was assessed as the difference in distribution of weight on the hind paws, which was measured using an incapacitance tester (Linton Instrumentation, Diss, Norfolk, UK) as previously described (Clayton et al., 2002; Elmes et al., 2005). The incapacitance tester consists of two sensitive strain gauge transducers, which measures the weight bearing on hind paws over a 3 s period. Differences in weight bearing between the inflamed and non-inflamed paw provide an index of basal hyperalgesia. This method of assessing inflammatory pain was employed in this study because there is mounting evidence that an over-reliance on measures of hypersensitivity based on evoked reflex responses, such as mechanical allodynia and hyperalgesia, and thermal hyperalgesia, may contribute to the lack of translational value of the outcomes of experimental pain studies to the development of clinically useful analgesics (Rice et al., 2008; Mogil, 2009). Weight bearing measurements (means of 3 at each time point) were taken 30 min before the final injection of URB597 or vehicle, 20 min after injection of URB597 or vehicle and then at hourly intervals following intraplantar injection of carrageenan or vehicle.
The effects of URB597/vehicle on carrageenan-induced hyperalgesia were assessed for 3 h post-carrageenan injection. After the final measurement of weight bearing, the ipsilateral and contralateral paw volumes were measured with a plethysmometer (mean of three readings). Rats were then stunned and decapitated, and the ipsilateral and contralateral hind paw tissue, lumbar enlargement of the spinal cord and midbrain were rapidly dissected, frozen in liquid nitrogen and stored at −80°C. Samples were processed for the measurement of endocannabinoids and related NAEs. Experimenters were unaware of the treatments for the ex vivo studies.
Measurement of endocannabinoids and NAEs
A validated lipid extraction technique was employed, with some alterations (Richardson et al., 2007). Briefly, tissue was finely minced and homogenized in acetonitrile (Fisher Scientific UK, Loughborough, UK) with 420 pmol of deuterated AEA (AEA-d8) and 1 nmol of deuterated 2AG (2AG-d8) internal standards (Cayman Europe, Tallinn, Estonia). The homogenized mixture was centrifuged (3200×g for 15 min, repeated twice), the supernatant collected, the solvent was evaporated and the residue was reconstituted in 200 µL acetonitrile (Fisher Scientific, Loughborough, UK). AEA, oleoyl ethanolamide (OEA), palmitoyl ethanolamide (PEA) and 2AG were measured simultaneously by LC-MS/MS. Analytes were separated chromatographically using a Waters Symmetry C18 column (100 × 2.1 mm internal diameter, 3.5 µm particle size; Hertfordshire, UK) with a mobile phase flowing at 0.3 mL·min−1, using a gradient elution with mobile phases consisting of solvents A (water, 1 g·L−1 ammonium acetate, 0.1% formic acid) and B (acetonitrile, 1 g·L−1 ammonium acetate, 0.1% formic acid). Analytes were injected from a cooled autosampler maintained at 4°C. Analysis was carried out using an Agilent 1100LC system (Agilent Technologies, Böblingen, Germany) coupled to a triple quadrupole Quattro Ultima mass spectrometer (Waters Ltd, Manchester, UK) recording in electrospray positive mode. Compounds were identified using the mass to charge (m/z) ratios of precursor and product ions. The lower limit of quantification (in 0.1 g of tissue) of AEA, PEA and OEA was 2.5 pmol·g−1 whilst that of 2AG was 50 pmol·g−1.
Gene and protein expression
The requirement for larger amounts of tissue in order to quantify endocannabinoid levels meant that there was insufficient lumbar spinal cord tissue from this first study for the investigation of changes in gene and protein expression. Thus, the study was repeated, as described above, and following the measurement of behavioural hyperalgesia, lumbar spinal cord tissue was again collected 3 h after carrageenan. Treatment groups were: repeated URB597 treatment plus hind paw carrageenan (n= 5); repeated vehicle treatment plus hind paw carrageenan (n= 6); acute URB597 plus hind paw carrageenan (n= 6), acute vehicle plus hind paw carrageenan (n= 5), repeated URB597 treatment plus hind paw saline (n= 5); repeated vehicle treatment plus hind paw saline (n= 5); acute URB597 plus hind paw saline (n= 5), acute vehicle plus hind paw saline (n= 5). In a separate study, the contribution of the cannabinoid CB1, versus PPARα, receptor systems to the inhibitory effects of a single dose of URB597 was also studied. The ability of systemic pre-administration of the CB1 receptor antagonist AM251 (5 mg·kg−1, n= 5) versus the PPARα antagonist GW6471 (10 mg·kg−1, n= 5) to attenuate the effects of single systemic administration of URB597 (n= 5) on carrageenan-induced pain behaviour was compared with vehicle carrageenan treated rats (n= 5). AM251 and GW6471 (Tocris Bioscience, Abingdon, UK) were dissolved in 0.3% Tween 20/ saline. The doses of antagonists were based on previous studies (Cuzzocrea et al., 2008; Sticht et al., 2011). Endocannabinoid levels were also measured in a subgroup of samples to ensure consistency between studies.
RNA extraction and cDNA synthesis
Approximately 50 mg of frozen tissue was homogenized in 2 mL of ice-cold Tri-reagent (Sigma-Aldrich, Gillingham, Dorset, UK) according to the manufacturer's instructions. Total RNA clean-up and on-column DNAse digestion was performed using RNeasy purification columns (Qiagen, Crawley, West Sussex, UK). RNA concentration and purity were determined using a Nanodrop spectrophotometer. For cDNA synthesis, 1 µg of total RNA was reverse-transcribed using superscript III reverse transcriptase (Invitrogen, Paisely, UK) in a total volume of 20 µL for 1 h at 50°C, and the reaction was terminated at 70°C for 15 min. The final concentration of cDNA was 50 ng·µL−1.
Taqman quantitative real-time PCR
The relative standard curve method based on Taqman quantitative real-time PCR (qRT-PCR) was used for quantifying gene expression. Samples were prepared in a total reaction volume of 25 µL [13 µL Taqman 2× reagent, 0.75 µL forward primer (10 µM), 0.75 µL reverse primer (10 µM), 0.5 µL Probe (10 µM), 5 µL water, and 5 µL cDNA]. qRT-PCR was performed using the StepOne Plus sequence detection system (Applied Biosciences, Warrington, UK). Gene expression was determined relative to β-actin.
Primers and probes for all genes (Table 1) were designed using Primer 3 software (Applied Biosystems, Warringon, UK) or obtained from published work and synthesized by MWG Biotech (Ebersberg, Germany).
Table 1.
List of gene primer and probe sequences
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| FAAH* | gcctcaaggaatgcttcagc | tgccctcattcaggctcaag | acaagggccacgactccacactgg |
| MAGL | tgccatctccatcctagcag | caaggatatgtttggcagga | atccggaatctgcatcgactttga |
| NAPE PLD | tcaagctcctctttggaacc | ctggaggaggacgtaaccaa | tatcccaaacgtgctcagatggct |
| COX-2** | ggcacaaatatgatgttcgca | cctcgcttctgatctgtcttga | tctttgcccagcacttcactcatcagttt |
| iNOS | cccagagtctctagacctcaacaaaca | gccctcgaaggtgagttgaa | aagtccagccgcaccaccctcc |
| IL-1ß∼ | cctctcaagcagagcacag | gggttccatggtgaagtcaac | tgtcccgaccattgctgtttcctagg |
| mPGES-1# | gcgaactgggccagaaca | ggcctacctgggcaaaatg | ccccggagcgaatgcgtgg |
| β-actin## | aggccatgtacgtagccatcca | tctccggagtccatcacaatg | tgtccctgtatgcctctggtcgtaccac |
Western blotting
Approximately 50 mg of tissue was homogenized in 1 mL of RIPA lysis buffer [150 mM NaCl, 25 mM Tris–HCl, pH 7.6, 0.5% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM Na3VO4 10 mM NaF, 1 × Complete Protease inhibitor (Roche Applied Sciences, Burgess Hill, West Sussex, UK)]. The homogenate was placed on a rotating wheel for 45 min at 4°C followed by centrifugation at 15 000×g for 20 min. The supernatant layer was then separated from the pellet and assayed for total protein concentration using a Pierce (Loughborough, UK) kit assay following the manufacturer's instructions.
25–50 µg of protein was separated on a 10% SDS-PAGE. The protein was transferred onto a nitrocellulose membrane and incubated overnight at 4°C with either rabbit polyclonal primary antibody to FAAH, monoacyl glycerol lipase (MAGL) or NAPE-PLD (Cayman, Cambridge Biosciences, UK; 1:200 dilution) and mouse monoclonal primary antibody to β-actin (Sigma; 1:5000). Blots were washed in TBS/Tween (TBST) buffer and incubated with IRDye® conjugated goat polyclonal anti-rabbit or anti-mouse IgG (LI-COR® Biosciences, Abingdon Park, Oxford, UK; 1:10 000 dilution) as appropriate. Scanning and densitometric analysis of blots was performed using a LI-COR® ODYSSEY infrared imaging system.
Measurement of FAAH activity
Treatment groups were as described above. FAAH activity was assessed as reported in Jhaveri et al. (2008). Enzyme in total particulate preparations (prepared by two successive centrifugations at 30 000 ×g for 30 min) was measured in the presence of 1, 5 and 20 µM N-arachidonoyl-[3H]-ethanolamine ([3H]-AEA, American Radiolabelled Chemicals, St Louis, MO, USA), using the equivalent of 0.5-1 mg original weight, incubated for 30 min at 37°C in 200 µL Tris EDTA, pH 7.4 buffer, halting the reaction with 400 µL activated charcoal (8% w/v) in 0.5 M HCl. [3H]-Ethanolamine in the supernatant layer following centrifugation (13 000 ×g for 5 min) was quantified by liquid scintillation counting. Non-FAAH hydrolytic activity (defined by pre-incubation of preparations for 20 min in the presence of 1 µM URB597) was not different from background. Although some of the FAAH inhibitors, including URB597, have off-target effects, these are associated with the liver (Zhang et al., 2007; Ahn et al., 2009).
Data analysis
Data are expressed as mean ± SEM or median ± range. Two-way anova, with a Bonferroni's post hoc test, was used to compare weight-bearing data between different treatment groups at different time points, as well as FAAH activity data. Km and Vmax values for FAAH activity in individual samples were calculated using non-linear regression fitting to a rectangular hyperbola using Prism (GraphPad, San Diego, CA). Comparison of levels of endocannabinoids was carried out using a non-parametric Kruskal–Wallis test and Dunn's post hoc test. All protein and mRNA data were analysed using one-way anova with a Bonferroni's selected pair post hoc test. The level of statistical significance levels was set at P < 0.05.
Materials
URB597, OEA, and PEA were from Cambridge Bioscience (Cambridge, UK); AEA from Tocris Bioscience, UK and activated charcoal from Sigma-Aldrich, UK.
Results
Acute, but not repeated, administration of URB597, attenuates inflammatory pain behaviour
Intraplantar injection of carrageenan resulted in a significant decrease in weight bearing on the ipsilateral hind paw, indicative of hyperalgesia (Figure 1) and an increase in paw volume (change in paw volume for acute vehicle carrageenan: 0.82 ± 0.05 mL; repeated vehicle carrageenan: 0.76 ± 0.13 mL). Acute treatment with the FAAH inhibitor URB597 (0.3 mg·kg−1) significantly attenuated the carrageenan-induced hyperalgesia at 120 and 180 min post-carrageenan injection (Figure 1A), but did not alter carrageenan-induced increases in hind paw volume (change in paw volume for acute URB597 carrageenan: 0.63 ± 0.06 mL). The marked inhibitory effects of acute URB597 at 120 min were significantly attenuated by pre-administration of the selective PPARα antagonist GW6471 and by the CB1 cannabinoid receptor antagonist AM251 (Figure 1B). In contrast to the inhibitory effects of acute treatment, repeated administration of URB597 (0.3 mg·kg−1, over 4 days) did not attenuate carrageenan-induced hyperalgesia at any time point (Figure 1C), nor carrageenan-induced increases in hind paw volume (change in paw volume for repeated URB597 carrageenan: 0.68 ± 0.13 mL).
Figure 1.
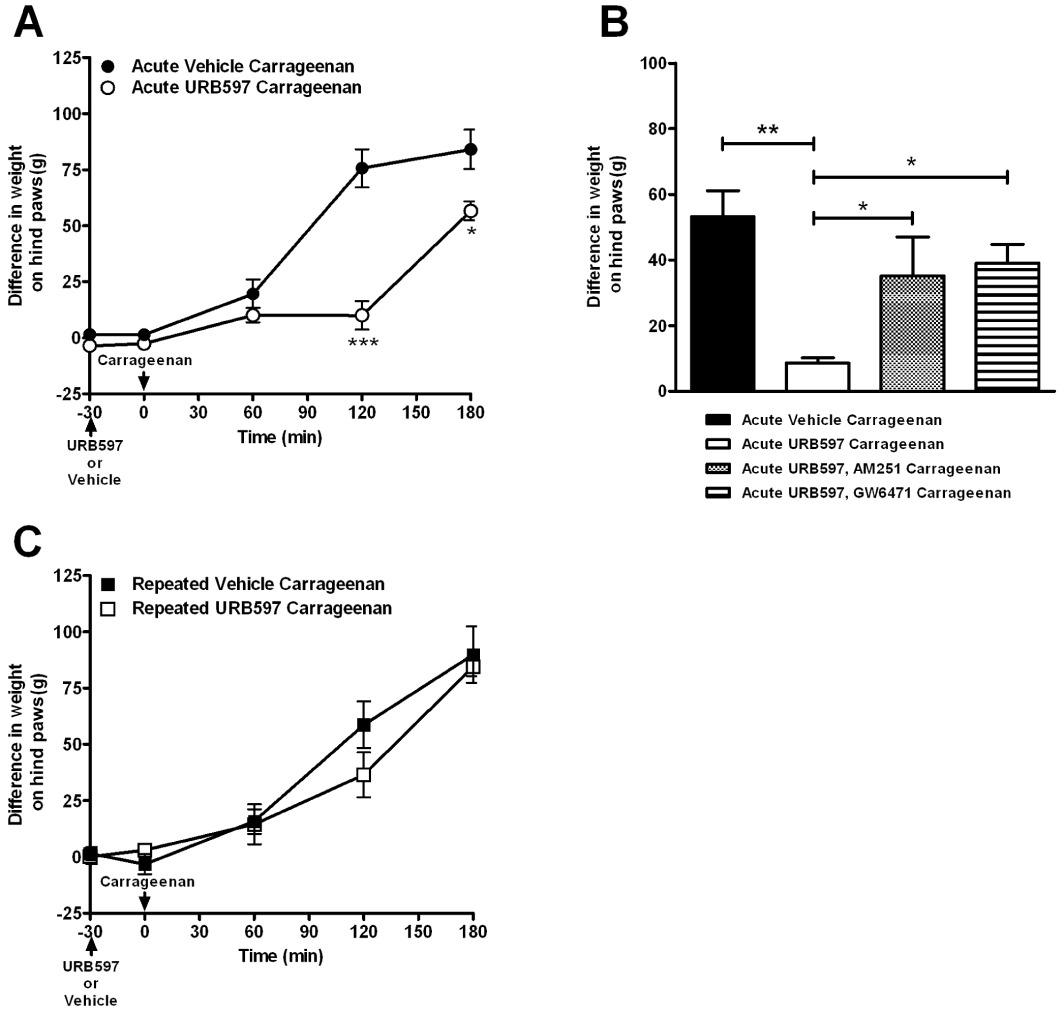
Comparison of the effects of acute and repeated administration of the FAAH inhibitor URB597 (0.3 mg·kg−1) on carrageenan-induced pain behaviour. (A) Acute URB597 attenuated carrageenan-induced changes in weight bearing. Data were analysed using two-way anova followed by Bonferroni's post hoc test, *P < 0.05, ***P < 0.001. (B) Inhibitory effects of acute URB597 at 120 min after carrageenan injection were significantly blocked by co-administration of the PPARα antagonist GW6471 (10 mg·kg−1) with URB597 and by co-administration of the CB1 receptor antagonist AM251 (5 mg·kg−1). Data were analysed using one-way anova with Bonferroni's post hoc test, *P < 0.05, **P < 0.01. (C) Repeated administration of URB597 did not alter carrageenan-induced changes in weight bearing. Data shown are differences in weight bearing between contralateral and ipsilateral hind paw and are expressed as mean ± SEM.
Acute and repeated administration of URB597 attenuates FAAH activity
In order to determine whether the differences in the effects of URB597 on pain behaviour were due to a loss of its inhibitory action at FAAH, the effects of acute and repeated administration of URB597 on FAAH activity were determined in spinal cord homogenates. FAAH activity was comparable in rats which received intraplantar injection of saline (5.4 ± 0.8 pmol·g−1·tissue·min−1) and carrageenan (4.7 ± 0.4 pmol·g−1·tissue·min−1). Both acute and repeated treatment with URB597 reduced FAAH activity to the same extent (acute: 0.6 pmol·g−1·tissue·min−1, 12 ± 2% of control, n= 7; repeated: 0.7 pmol·g−1·tissue·min−1, 16 ± 3% of control, n= 6, P= 0.31 Mann–Whitney t-test). Thus, the two treatment strategies with URB597 produced comparable inhibition of FAAH activity in the spinal cord and therefore changes in the pharmacokinetics or activity of URB597 following repeated administration were unlikely to account for the differences in the effects of URB597 on pain behaviour.
Differential effects of acute and repeated administration of URB597 on levels of NAEs
To investigate further the basis for the reduced effectiveness of repeated administration of URB597 on pain behaviour, levels of the NAEs (AEA, PEA, OEA) and of 2-AG were measured in the ipsilateral spinal cord following acute and repeated treatment with URB597 in carrageenan-treated rats. Acute URB597 significantly elevated levels of AEA, PEA and OEA, but not 2-AG, in the ipsilateral spinal cord of carrageenan-treated rats compared with the control group (i.p. saline and intraplantar injection of carrageenan) (Figure 2). Repeated administration of URB597 did not significantly increase levels of AEA, PEA or OEA in the spinal cord of carrageenan-treated rats, compared with the control group (repeated i.p. saline and intraplantar injection of carrageenan), although there was a trend towards an increase (Figure 2). The magnitude of the elevations in NAEs in the ipsilateral spinal cord following repeated administration of URB597 was smaller than the effects of acute URB597 treatment (Table 2). It is noteworthy that when considering levels of AEA in the spinal cord following acute or repeated administration of vehicle in carrageenan-treated rats, there was a greater spread in the dataset from the repeated treatment group; however, these differences were not significant.
Figure 2.
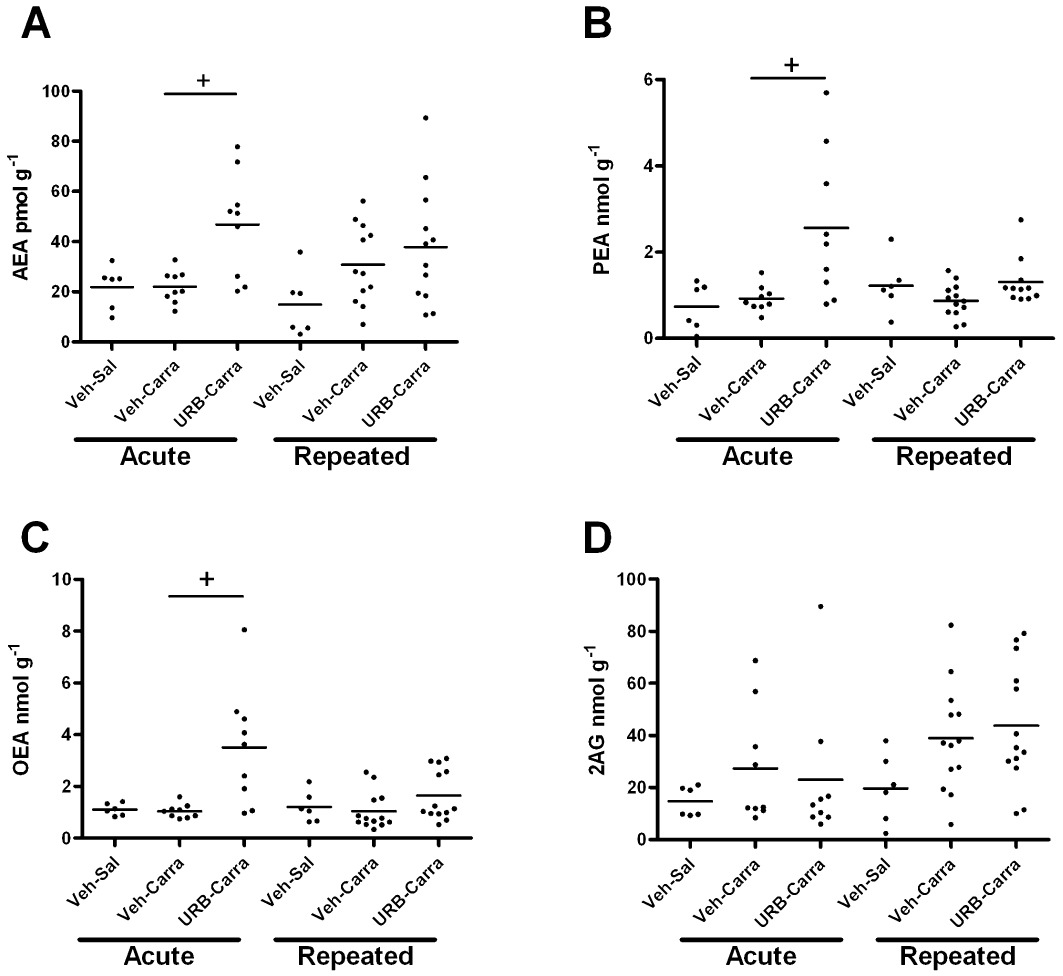
Effects of acute and repeated administration of URB597 or vehicle on intraplantar injection of carrageenan (carra)-, or saline (sal)-, induced changes in levels of (A) AEA, (B) PEA, (C) OEA and (D) 2-AG in the ipsilateral spinal cord 3 h following intraplantar injection (see Figure 1 legend for further details of drug treatments). Data are expressed as median and range (n= 9–13 rats per group) and were analysed using Kruskal–Wallis and Dunn's post hoc test: +P < 0.05, compared with vehicle carrageenan.
Table 2.
Comparison of the effects of acute and repeated administration of URB597 (0.3 mg·kg−1) on levels of AEA, PEA, OEA and 2-AG in the ipsilateral spinal cord of carrageenan-treated rats
| Relative levels of endocannabinoids and related NAEs (% control) | |||||
|---|---|---|---|---|---|
| AEA | PEA | OEA | 2-AG | ||
| Spinal Cord | Acute URB597 | 213 ± 31* | 278 ± 62 | 337 ± 72* | 84 ± 32 |
| Repeated URB597 | 123 ± 22 | 149 ± 17 | 158 ± 26 | 112 ± 17 | |
Data are expressed as a percentage of the mean level in the ipsilateral spinal cord of carrageenan-treated rats receiving either acute or repeated administration of vehicle (n= 9–13 rats per group). Statistical comparisons between the effects of acute and repeated administration of URB597 was carried out using a Mann–Whitney non-parametric test (*P < 0.05)
To evaluate further the potential site of action of URB597, the effects of systemically administered URB597 on levels of AEA, PEA and OEA were determined in the hind paw and the midbrain. Neither acute, nor repeated, treatment with systemic URB597 altered levels of the NAEs in the carrageenan-inflamed hind paw (Table 3). At the level of the midbrain, both acute and repeated administration of URB597 significantly elevated levels of AEA and PEA in carrageenan-treated rats (Table 3). Consistent with the observations in the spinal cord, the elevation of the NAEs in the midbrain was greater following acute than after repeated administration of URB597.
Table 3.
Levels of NAEs in the hind paw (A) and midbrain (B) of rats receiving an acute or repeated treatment with URB597 (0.3 mg·kg−1) or vehicle prior to intraplantar injection of carrageenan
| A: Hind paw | |||
|---|---|---|---|
| Treatment | AEA (pmol g−1) median (range) | OEA (nmol g−1) median (range) | PEA (nmol g−1) median (range) |
| Acute vehicle + carrageenan | 5.22 (4.34–6.89) | 0.12 (0.04–0.19) | 0.12 (0.05–0.34) |
| Acute URB597 + carrageenan | 6.47 (4.26–11.15) | 0.09 (0.07–0.17) | 0.14 (0.06–0.30) |
| Repeated vehicle + carrageenan | 8.50 (1.45–12.28) | 0.15 (0.06–0.28) | 0.18 (0.14–0.24) |
| Repeated URB597 + carrageenan | 4.14 (1.38–14.14) | 0.15 (0.07–0.19) | 0.13 (0.08–0.19) |
| B: Midbrain | |||
|---|---|---|---|
| Treatment | AEA pmol g−1 median (range) | OEA nmol g−1 median (range) | PEA nmol g−1 median (range) |
| Acute vehicle + carrageenan | 16.41 (14.0–34.98) | 0.85 (0.72–1.34) | 1.34 (0.83–1.58) |
| Acute URB597 + carrageenan | 29.2 (18.6–44.78)* | 2.97 (0.98–3.36)* | 3.21(1.30–3.89)* |
| Repeated vehicle + carrageenan | 11.69 (8.84–22.69) | 0.70 (0.32–0.75) | 0.73 (0.38–1.01) |
| Repeated URB597 + carrageenan | 23.27 (15.57–51.58)* | 1.83 (0.51–2.67) | 2.28 (0.67–3.71)* |
Data are expressed as median and range, n= 9–13 rats per group. Comparisons between the effects of acute vehicle and acute URB597 or repeated vehicle and repeated URB597 were performed using a Mann–Whitney non-parametric test, *P < 0.05, significantly different from vehicle + carrageenan.
Given that the inhibitions of FAAH activity were comparable after acute and repeated administration of URB597, the differences in the effects of acute versus repeated administration of URB597 on the levels of the NAEs suggests that sustained pharmacological blockade of FAAH may have modulated the synthesis of these mediators. Thus, we investigated whether inhibition of FAAH influenced the expression and protein levels of NAPE-PLD, the major synthetic enzyme for long chain NAEs. Both acute and repeated administration of URB597 were associated with marked decreases in the expression of NAPE-PLD protein in the ipsilateral spinal cord of carrageenan and saline-treated rats (Figure 3A, B). URB597 did not alter FAAH protein in the the ipsilateral spinal cord of carrageenan and saline-treated rats (Figure 3A). Neither NAPE-PLD, nor FAAH, mRNA expression was altered by either acute or repeated administration of URB597 (Figure 4). In keeping with the established divergence of catabolic pathways for the NAEs and 2-AG, mRNA (acute vehicle + carrageenan: 1.12 ± 0.12; acute URB + carrageenan: 1.10 ± 0.17, relative to β-actin) and protein (acute vehicle + carrageenan: 1.45 ± 0.16; acute URB + carrageenan: 1.59 ± 0.27, relative to β-actin) levels of the major 2-AG catabolic enzyme MAGL were comparable in the spinal cord. Similarly, repeated administration of URB597 did not alter levels of MAGL mRNA or protein in the spinal cord of saline or carrageenan-treated rats (data not shown).
Figure 3.
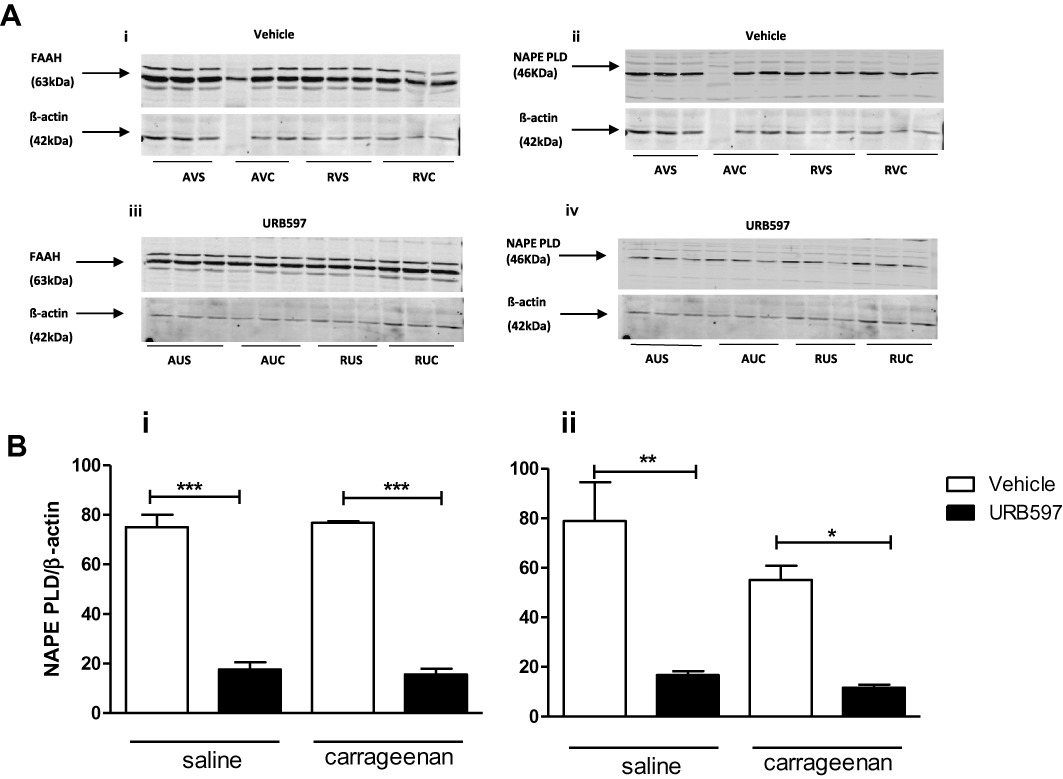
Effects of URB597 on FAAH and NAPE PLD protein expression in the ipsilateral spinal cord (see Figure 1 legend for further details of drug treatments). A (i, iii): Neither acute, nor repeated, URB597 altered FAAH protein levels. A (ii, iv), B (i, ii): Acute and repeated administration of URB597 reduced NAPE-PLD protein levels by approximately two-thirds, compared with the corresponding vehicle treated groups. AVS (Acute vehicle + saline), AUS (Acute URB597 + saline), AVC (Acute Vehicle + carrageenan), AUC (Acute URB597 + carrageenan), RVS (Repeated vehicle + saline), RUS (Repeated URB597 + saline), RVC (Repeated Vehicle + carrageenan), RUC (Repeated URB597 + carrageenan), n= 3, per each treatment group, performed in duplicate. Statistical analysis with one-way anova with a Bonferroni's post test, *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
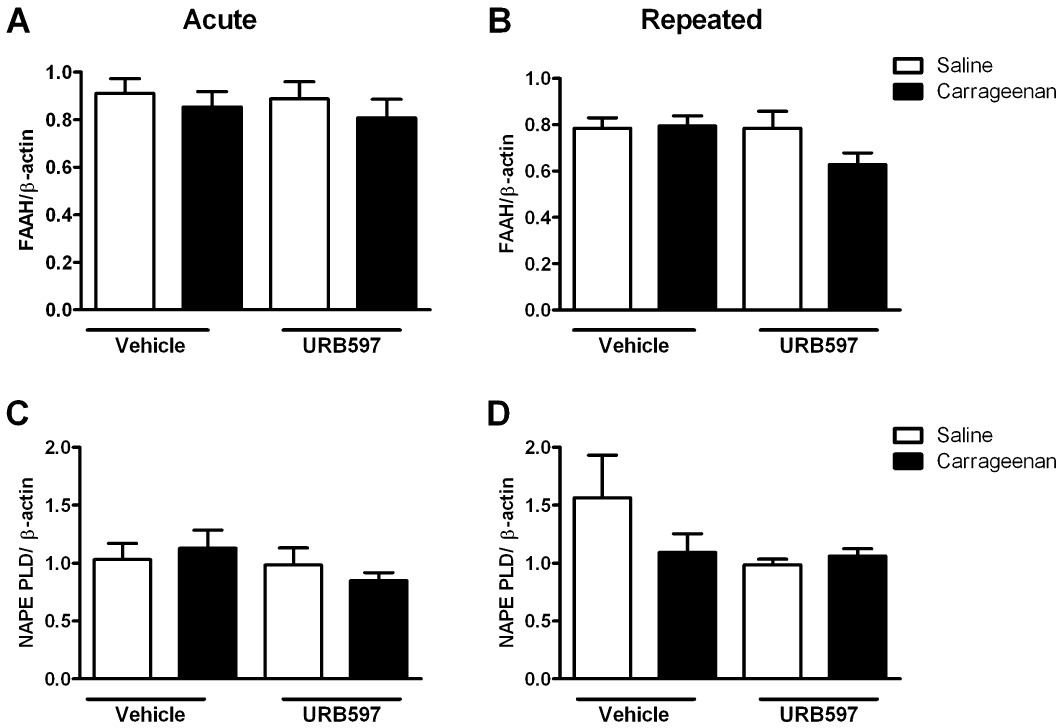
Effects of acute and repeated administration of URB597 on FAAH and NAPE-PLD mRNA expression in the ipsilateral spinal cord. (A and B) FAAH mRNA levels, relative to β-actin, in the ipsilateral spinal cord of saline and carrageenan-treated rats were not altered by acute or repeated administration of URB597. (C and D) NAPE PLD mRNA levels, relative to β-actin, in the ipsilateral spinal cord of saline and carrageenan-treated rats were not altered by acute or repeated administration of URB597 (n= 5 per treatment group).
Effects of acute and repeated administration of URB597 on pro-inflammatory spinal responses
As the spinal cord plays a critical role in the processing of noxious sensory inputs and our biochemical data demonstrate a spinal site of action of acute and repeated administration of URB597, the effects of URB597 on the spinal pro-inflammatory responses associated with inflammatory pain behaviour were determined. As described previously, carrageenan-induced hyperalgesia was associated with an increased expression of mRNA encoding COX-2 and mPGES-1 in the ipsilateral spinal cord, compared to rats receiving intraplantar injection of saline (Figure 5). Carrageenan-induced hyperalgesia was also associated with an increased expression of iNOS and IL1β mRNA in the ipsilateral spinal cord (Figure 5). Acute URB597 treatment significantly decreased the carrageenan-induced increases in spinal COX-2 (60 ± 14% decrease), mPGES-1 (65 ± 15% decrease), IL-1β (57 ± 5% decrease) and iNOS (67 ± 9% decrease) expression, compared with vehicle (Figure 5). Alongside the effects of acute URB597 on FAAH activity and endocannabinoid levels in the spinal cord, these data suggest that a spinal site of action contributes to the effects of acute systemically administered URB597 on behavioural hyperalgesia and that inhibition of FAAH attenuates pro-inflammatory spinal mechanisms. Repeated URB597 treatment had a smaller effect on carrageenan-induced COX-2 and mPGES-1 expression compared with the effect of acute URB597 (Figure 5). Although there were some small differences in the absolute levels of spinal COX-2, induced by carrageenan. following acute or repeated vehicle treatment, these were not significant and carrageenan-induced levels of mPGES-1 were comparable for both vehicle treatment groups. In contrast to COX-2, carrageenan-induced spinal IL1β and iNOS expression were significantly attenuated by repeated administration of URB597 (Figure 5). Indeed, both acute and repeated administration of URB597 abolished any effect of carrageenan on IL1β and iNOS expression in the spinal cord.
Figure 5.
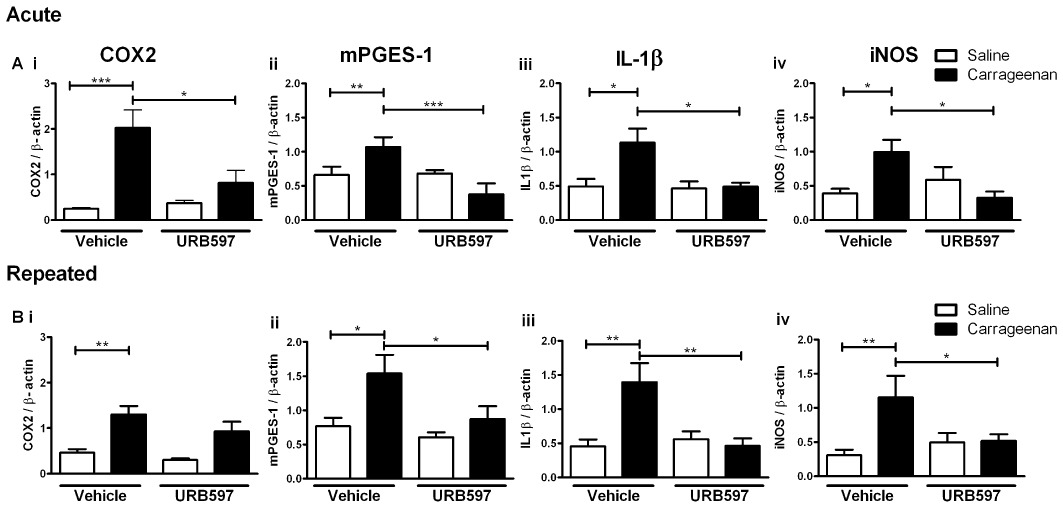
Effects of acute and repeated administration of URB597 on carrageenan induced pro-inflammatory gene expression in the ipsilateral spinal cord. Intraplantar injection of carrageenan produced a robust elevation in COX-2, mPGES-1, IL1β and iNOS mRNA in the ipsilateral spinal cord. Acute administration of URB597 significantly reduced carrageenan-induced pro-inflammatory gene expression. By contrast, repeated administration of URB597 did not significantly attenuate carrageenan-induced COX-2 expression in the spinal cord and had less effect on mPGES-1 expression compared with acute URB597. Repeated administration of URB597 attenuated carrageenan-induced IL1β and iNOS expression. Statistical analysis with one-way anova with Bonferroni's post hoc test *P < 0.05, **P < 0.01, ***P < 0.0001 (n= 4–6 per treatment group).
Discussion
The major finding of the present study is that repeated administration of URB597 did not attenuate inflammatory pain behaviour, in contrast to the inhibitory effects of a single dose of the FAAH inhibitor, which were significantly blocked by the PPARα-selective antagonist GW6471 and by the CB1 receptor-selective antagonist AM251. Both acute and repeated administration of URB597 inhibited FAAH activity in the spinal cord to a similar extent. Despite this, levels of AEA, PEA and OEA in the spinal cord were elevated to a greater extent by URB597 administered as a single dose, compared with repeated administration. Our data suggest that sustained pharmacological inhibition of FAAH results in an adaptation in the synthesis of the endocannabinoids, and related NAEs, or their catabolism by alternative pathways, which are not dependent upon FAAH (Kozak et al., 2002), in the spinal cord and are not evident following FAAH knockout. The demonstration that sustained pharmacological inhibition of FAAH does not mimic the robust inhibitory effects of FAAH knockout (see below) on inflammatory pain behaviour (both experimental approaches inhibit FAAH before the establishment of the pain state), indicates the importance of multiple experimental approaches in the identification of potential analgesic targets.
Comparison of the effects of acute versus repeated treatment with URB597 in the model of inflammatory pain revealed that peripheral hind paw levels of NAEs were unaltered by either treatment, suggesting that this is not a major site of action of URB597 following systemic administration. Furthermore, neither acute nor sustained inhibition of FAAH with URB597 altered carrageenan-induced increases in hind paw volume. These data suggest that the ability of acute URB597 treatment to attenuate the carrageenan-induced hyperalgesia is via mechanisms distinct from an anti-inflammatory action. Our data are consistent with the observation that higher doses of systemic URB597 (1 and 3 mg·kg−1) are required to attenuate carrageenan-induced increases in paw volume, whereas 0.3 mg·kg−1 URB597 attenuates carrageenan-induced thermal hyperalgesia without influencing paw volume (Costa et al., 2010). This dose of URB597 has, however, been shown to attenuate carrageenan-induced increases in paw volume in the anaesthetized mouse (Holt et al., 2005). Previously, we have shown that local hind paw injection of URB597 can elevate levels of NAEs and attenuate pain behaviour but does not alter paw volume in this model (Jhaveri et al., 2008).
Acute administration of URB597 significantly elevated levels of NAEs in both the spinal cord and midbrain, which is consistent with the original report that this dose of URB597 produces a maximal inhibition of basal AEA hydrolysis in the brain from 15 min to 6 h after administration and elevates brain levels of AEA and PEA from 1–6 h (Fegley et al., 2005). In contrast to the effects of acute URB597, levels of NAEs in the spinal cord were not altered following repeated administration of URB597 but were elevated in the midbrain. The demonstration of tissue-specific differences in the effects of URB597 is consistent with earlier reports (Gobbi et al., 2005; Bortolato et al., 2007; Long et al., 2011). For example, 5 weeks of daily administration of URB597 increased levels of AEA in some brain regions; however, despite hippocampal FAAH activity being significantly reduced, levels of AEA were unaltered (Bortolato et al., 2007). In our study, acute and repeated treatment with URB597 produced a comparable inhibition of hydrolytic activity; thus, it is unlikely that the lack of effect of repeated URB597 treatment on pain behaviour and levels of NAEs in the spinal cord reflects an inadequate blockade of enzyme activity at this level. This notion is further supported by the ability of repeated URB597 treatment to elevate levels of NAEs in the midbrain. The differences in the behavioural effects of sustained pharmacological inhibition of FAAH, compared to FAAH knockout,, on inflammatory pain responses (Lichtman et al., 2004; Naidu et al., 2010), suggest that the endocannabinoid system may influence the development of pain circuits that are subject to considerable neonatal and postnatal influence (Fitzgerald, 2005), as well as possible effects on other neuronal systems which affect inflammatory pain pathways.
There are marked differences between the effects of pharmacological intervention with URB597 in models of inflammatory and neuropathic pain, which are likely to reflect differences in the mechanisms underlying these different pain states, or differences in the behavioural tests employed (changes in weight bearing versus stimulus-evoked nociceptive responses, and the modality of stimulus). Indeed, the original report of the effects of pharmacological inhibition of FAAH in inflammatory and neuropathic rats indicated a lack of effect of URB597 on mechanical allodynia in neuropathic rats but a pronounced inhibitory effect on thermal hyperalgesia in an inflammatory pain model (Jayamanne et al., 2006). Repeated (4 day) oral administration of a far higher dose of URB597 in mice did, however, attenuate established neuropathic pain behaviour via a CB1 receptor-dependent mechanism (Russo et al., 2007). The more recent description of the dose–response effects of URB597 on neuropathic pain behaviour in mice demonstrates that the lower doses of URB597, which inhibit inflammatory pain behaviour in rats (present study, Jayamanne et al., 2006) and mice (Costa et al., 2010), do not alter mechanical allodynia in neuropathic mice and that far higher doses are required to inhibit these responses with both oral and i.p. routes of administration (Kinsey et al., 2009). These data suggest that differences in the mechanisms underlying acute inflammatory pain responses from those underlying nerve damage-induced pain, and the effect these pain states have on the endocannabinoid system per se (Sagar et al., 2009), may account for the differences in efficacy of URB597 in these two different pain states.
In the present study, the inhibitory effects of a single dose of URB597 were significantly blocked by a PPARα-selective antagonist, which is consistent with our earlier work (Jhaveri et al., 2008; Sagar et al., 2008), as well as a CB1 receptor-selective antagonist. These data are consistent with FAAH metabolizing a number of NAEs which have multiple targets, including PPARα, CB1 receptors and CB2 receptors, which modulate inflammatory pain responses. Although not investigated in this study, CB2 receptors may also contribute to the inhibitory effects of URB597 seen in this model of inflammatory pain (Jayamanne et al., 2006). Previous studies have shown that PEA, a ligand for the nuclear receptor PPARα, has rapid anti-inflammatory and analgesic effects (LoVerme et al., 2005; 2006). PEA has also been reported to have anti-nociceptive effects in a neuropathic pain model, apparently via activation of CB1, PPARγ and TRPV1 receptors (Costa et al., 2008). Thus, the reduced effectiveness of repeated administration of URB597 to elevate spinal levels of PEA may indeed contribute to the lack of behavioural analgesia seen with this treatment. It is feasible that secondary changes in receptor levels, for example CB1 or PPARα down-regulation, as a result of a sustained elevation in NAEs contributes to the lack of effect of repeated administration of URB597 on pain behaviour. Equally, effects of URB597 on levels of endovanilloids and the activation state of TRPV1 may also affect our data (Maione et al., 2006). There is, however, no evidence for these, or other, changes in receptor function occurring in FAAH knockout mice, which have elevated levels of NAEs for a prolonged period of time.
On the basis that there were differential effects of URB597 treatment on pain behaviour and levels of NAEs in the spinal cord, but not in the attenuation of hydrolytic activity or FAAH expression, we investigated whether this treatment might influence the synthesis of the NAEs by the best characterized NAE synthetic enzyme, NAPE-PLD (Wang and Ueda, 2009). Single and repeated administration of URB597 reduced NAPE-PLD protein, but not mRNA, expression in the spinal cord, suggesting that changes in protein levels arose as a result of translational or post-translational mechanism(s). The functional effect of acute and repeated administration of URB597 on NAPE-PLD and NAE levels should be considered in the context of NAE turnover. A single dose of URB597 increases NAE levels for at least 6 h after treatment (Fegley et al., 2005); thus, decreased NAPE-PLD protein levels must take six or more hours to affect NAE levels. On this basis, it is feasible that it is only with the repeated dosing strategy over a longer period of time that the reduced NAPE-PLD protein levels affect the rate of NAE synthesis and have a physiological effect. Indeed, if the rate of NAE synthesis is lower in the repeated treatment group, then the effects of inhibition of FAAH in terms of increasing levels of NAEs would be less pronounced. It is important to bear in mind that the contribution of the NAPE-PLD pathway, relative to other synthetic routes of synthesis (Ueda et al., 2010), to the generation of NAEs has not been widely studied. Genetic deletion of NAPE-PLD decreases basal brain levels of saturated long chain NAEs (including PEA), but not unsaturated NAEs (including AEA; Leung et al., 2006). Thus, if NAPE-PLD contributes in a similar manner to stimulated levels of NAEs, the reported decrease in protein levels of NAPE-PLD would be predicted to have a greater influence on the levels of PEA than on those of AEA.
Previously, we have reported an increased expression of spinal cord NAPE-PLD protein, but not mRNA, in a chronic model of osteoarthritic pain (Sagar et al., 2010). However, in this shorter term, carrageenan-induced, model of inflammatory pain neither NAPE-PLD mRNA nor protein levels in the spinal cord were altered. Inflammatory signalling decreased NAPE-PLD mRNA and protein levels locally at the site of injury (Marquez et al., 2009). LPS, which, like carrageenan, stimulates Toll-like receptor 4, has been proposed to cause an increased histone deacetylase activity, leading to decreased NAPE-PLD mRNA and protein levels in RAW264.7 macrophages (Zhu et al., 2011). The demonstration that NAPE-PLD expression is regulated in a tissue and stimulus-specific manner, and that this pathway is influenced by FAAH inhibitors, supports the further investigation of the transcriptional and post-transcriptional control of NAPE-PLD.
The spinal activation of pro-inflammatory signalling pathways, in particular the induction of COX-2 and PGE2 production plays a critical role in inflammatory pain states (Samad et al., 2001). Thus, we investigated whether the differential effects of acute versus repeated URB597 treatment on pain behaviour reflected differences in the ability of URB597 to modulate pro-inflammatory signalling at this level. Despite the lack of effect of repeated URB597 treatment on pain behaviour, both acute and repeated URB597 treatment attenuated carrageenan-induced pro-inflammatory signalling at the level of the spinal cord, in particular iNOS and IL1β induction. These data suggest that iNOS and/or IL1β have a supporting, but not essential, role in these models of inflammatory pain which is consistent with the report that genetic disruption of iNOS (Tao et al., 2003) or IL1β (Fantuzzi and Dinarello, 1996) did not alter inflammatory responses to carrageenan, or LPS respectively. The effects of acute and repeated administration of URB597 on carrageenan-induced expression of COX-2 and mPGES-1 were, however, less clear-cut. Acute URB597 produced a robust inhibition of carrageenan-induced COX-2 and mPGES-1 gene induction. Repeated administration of URB597 had less robust inhibitory effects on carrageenan-induced COX-2 and mPGES-1 gene induction, but there was a tendency for these to be inhibited. Small differences in the absolute levels of carrageenan-induced COX-2 induction in the control groups (acute saline vs. repeated saline), which probably reflected the biological variation associated with these types of complex in vivo studies, prevented further conclusions being drawn.
In conclusion, we have demonstrated that repeated administration of low-dose URB597 did not produce a behavioural analgesia, in contrast to the robust inhibitory effect of acute URB597 treatment. These behavioural effects were consistent with the differential effect of acute and repeated URB597 treatment on levels of NAEs in the spinal cord, but not the midbrain, but did not reflect differences in their ability to attenuate FAAH activity. Our data support the accumulating evidence that there are tissue-specific differences in the synthetic and catabolic pathways that regulate levels of NAEs, which may have further significance under pathological conditions, such as the spinal processing of inflammatory pain.
Acknowledgments
BN Okine, LM Norris, S Woodhams and A Patel were funded by MRC DTA studentships. This work was supported by project grant funding from the Wellcome Trust.
Glossary
- 2-AG
2-arachidonoylglycerol
- AEA
arachidonoyl ethanolamide (anandamide)
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- FAAH
fatty acid amide hydrolase
- GW6471
[(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl) phenyl]-1-propenyl] amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxazolyl) ethoxy] phenyl] propyl]-carbamic acid ethyl ester
- MAGL
monoacylglycerol lipase
- NAE
N-acylethanolamine
- mPGES-1
microsomal PGE synthase-1
- NAPE-PLD
N-acylphosphatidylethanolamine phospholipase D: OEA, oleoyl ethanolamide
- PEA
palmitoyl ethanolamide
- TRPV1
transient receptor potential vanilloid type 1
- URB597
cyclohexyl carbamic acid 3′-carbamoyl- biphenyl-3-yl ester
- WY14643
chloro-6-(2, 3-xylidino)-2-pyrimidinylthioacetic acid
Conflicts of interest
There are no conflicts of interest to be declared.
References
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol Pharmacol. 2009;60:119–125. [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic Pathways That Regulate Endocannabinoid Signaling in the Nervous System. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (5th Edition) 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claveau D, Sirinyan M, Guay J, Gordon R, Chan CC, Bureau Y, et al. Microsomal prostaglandin E synthase-1 is a major terminal synthase that is selectively up-regulated during cyclooxygenase-2-dependent prostaglandin E2 production in the rat adjuvant-induced arthritis model. Immunol. 2003;170:4738–4744. doi: 10.4049/jimmunol.170.9.4738. [DOI] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O'Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARÎ3 receptors and neurotrophic factors. Pain. 2008;139:541–550. doi: 10.1016/j.pain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Costa B, Bettoni I, Petrosino S, Comelli F, Giagnoni G, Di Marzo V. The dual fatty acid amide hydrolase/TRPV1 blocker, N-arachidonoyl-serotonin, relieves carrageenan-induced inflammation and hyperalgesia in mice. Pharmacol Res. 2010;61:537–546. doi: 10.1016/j.phrs.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci U S A. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Bruscoli S, Mazzon E, Crisafulli C, Donato V, Di Paola R, et al. Peroxisome proliferator-activated receptor-alpha contributes to the anti-inflammatory activity of glucocorticoids. Mol Pharmacol. 2008;73:323–337. doi: 10.1124/mol.107.041475. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, et al. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Erhuma A, Salter AM, Sculley DV, Langley-Evans SC, Bennett AJ. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am J Physiol Endocrinol Metab. 2007;292:E1702–E1714. doi: 10.1152/ajpendo.00605.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G, Dinarello CA. The inflammatory response in interleukin-1 beta-deficient mice: comparison with other cytokine-related knock-out mice. J Leukoc Biol. 1996;59:489–493. doi: 10.1002/jlb.59.4.489. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay J, Bateman K, Gordon R, Mancini J, Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin E2 (PGE2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- Hay C, de Belleroche J. Carrageenan-induced hyperalgesia is associated with increased cyclo-oxygenase-2 expression in spinal cord. Neuroreport. 1997;8:1249–1251. doi: 10.1097/00001756-199703240-00038. [DOI] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Robinson I, Garle MJ, Patel A, Sun Y, et al. Inhibition of fatty acid amide hydrolase and cyclooxygenase-2 increases levels of endocannabinoid related molecules and produces analgesia via peroxisome proliferator-activated receptor-alpha in a model of inflammatory pain. Neuropharmacology. 2008;55:85–93. doi: 10.1016/j.neuropharm.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, et al. Fatty acid amide hydrolase inhibition heightens anandamide signalling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbarz MJ, Luo L, Chang L, Tham CS, Palmer JA, Wilson SJ, et al. Biochemical and biological properties of 4-(3-phenyl-[1,2,4] thiadiazol-5-yl)-piperazine-1-carboxylic acid phenylamide, a mechanism-based inhibitor of fatty acid amide hydrolase. Anesth Analg. 2009;108:316–329. doi: 10.1213/ane.0b013e31818c7cbd. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. Blockade of Endocannabinoid-Degrading Enzymes Attenuates Neuropathic Pain. J Pharmacol Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Long JZ, LaCava M, Jin X, Cravatt BF. An anatomical and temporal portrait of physiological substrates for fatty acid amide hydrolase. J Lipid Res. 2011;52:337–344. doi: 10.1194/jlr.M012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Marquez L, Suarez J, Iglesias M, Bermudez-Silva FJ, Rodriguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE. 2009;4:e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between Enzyme Inhibitors of Fatty Acid Amide Hydrolase and Cyclooxygenase in Visceral Nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu PS, Kinsey SG, Guo TL, Cravatt BF, Lichtman AH. Regulation of inflammatory pain by inhibition of fatty acid amide hydrolase. J Pharmacol Exp Ther. 2010;334:182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, et al. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Rioja I, Bush KA, Buckton JB, Dickson MC, Life PF. Joint cytokine quantification in two rodent arthritis models: kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatment. Clin Exp Immunol. 2004;137:65–73. doi: 10.1111/j.1365-2249.2004.02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Sagar DR, Kendall DA, Chapman V. Inhibition of fatty acid amide hydrolase produces PPAR-alpha-mediated analgesia in a rat model of inflammatory pain. Br J Pharmacol. 2008;155:1297–1306. doi: 10.1038/bjp.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Gaw AG, Okine BN, Woodhams SG, Wong A, Kendall DA, et al. Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain. 2009;5:59–72. doi: 10.1186/1744-8069-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, et al. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 2010;62:3666–3676. doi: 10.1002/art.27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1[beta]-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Shafer LM, Slice LW. Anisomycin induces COX-2 mRNA expression through p38 (MAPK) and CREB independent of small GTPases in intestinal epithelial cells. Biochim Biophys Acta. 2005;1745:393–340. doi: 10.1016/j.bbamcr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Song MJ, Wang YQ, Wu GC. Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull. 2009;78:335–341. doi: 10.1016/j.brainresbull.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Sticht MA, Long JZ, Rock EM, Limebeer CL, Mechoulam R, Cravatt BF, et al. The MAGL inhibitor, JZL184, attenuates LiCl-induced vomiting in the Suncus murinus and 2AG attenuates LiCl-induced nausea-like behavior in rats. Br J Pharmacol. 2011;165:2425–2435. doi: 10.1111/j.1476-5381.2011.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Tao YX, Mao P, Zhao C, Li D, Liaw WJ, et al. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience. 2003;120:847–854. doi: 10.1016/s0306-4522(03)00362-2. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat. 2009;89:112–119. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang D, Saraf A, Kolasa T, Bhatia P, Zheng GZ, Patel M, et al. Fatty acid amide hydrolase inhibitors display broad selectivity and inhibit multiple carboxylesterases as off-targets. Neuropharmacology. 2007;52:1095–1105. doi: 10.1016/j.neuropharm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, et al. Proinflammatory stimuli control NAPE-PLD expression in macrophages. Mol Pharmacol. 2011;79:786–792. doi: 10.1124/mol.110.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]


