Abstract
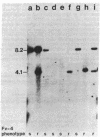
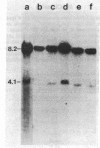
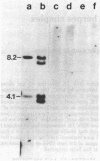
Several strains of laboratory and wild-derived mice from Japan carry the dominant allele at the Fv-4 locus (Fv-4r) that is responsible for resistance to infection by exogenous ecotropic murine leukemia virus. We have used blot hybridization with a probe specific for the ecotropic viral envelope to show that a unique envelope-reactive sequence is present in the Japanese mouse Mus musculus molossinus and in four independently derived partially congeneic strains carrying Fv-4r. Analysis of 31 backcross mice shows complete concordance between inheritance of this fragment and resistance to Friend virus complex-induced erythroblastosis. Inheritance of this sequence also suppresses spontaneous expression of the endogenous ecotropic viruses carried by M. m. molossinus. Restriction enzyme analysis shows that the Fv-4r-associated sequence is different from the full-length ecotropic proviruses of laboratory mice. Infectious virus cannot be induced from mice carrying only the Fv-4r-associated sequence. Examination of other wild-derived mice resistant to Friend virus complex shows that Mus cervicolor cervicolor also contains the Fv-4r sequence. Our data indicate that a unique or incomplete provirus containing ecotropic envelope-related sequences is responsible for Fv-4-mediated resistance to both exogenous and endogenous ecotropic virus in various Asian mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler C. E., Staal S. P., Rowe W. P., Martin M. A. Variation in the number of copies and in the genomic organization of ecotropic murine leukemia virus proviral sequences in sublines of AKR mice. J Virol. 1982 Aug;43(2):629–640. doi: 10.1128/jvi.43.2.629-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Klement V., Rongey R. R., McConahey P., Estes J. D., Huebner R. J. Type C virus expression in lymphoma-paralysis-prone wild mice. J Natl Cancer Inst. 1976 Sep;57(3):585–590. doi: 10.1093/jnci/57.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S., Pal B. K., Estes J. D., O'Brien S. J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc Natl Acad Sci U S A. 1980 Jan;77(1):531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Oie H., Lalley P., Moss W. W., Minna J. D. Identification of mouse chromosomes required for murine leukemia virus replication. Cell. 1977 Aug;11(4):949–956. doi: 10.1016/0092-8674(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Odaka T. Cellular expression of murine leukemia virus gp70-related antigen on thymocytes of uninfected mice correlates with Fv-4 gene-controlled resistance to Friend leukemia virus infection. Virology. 1983 Jul 15;128(1):127–139. doi: 10.1016/0042-6822(83)90324-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Sato H., Odaka T. Mapping of the Fv-4 mouse gene controlling resistance to murine leukemia viruses. Int J Cancer. 1981 Aug 15;28(2):237–240. doi: 10.1002/ijc.2910280218. [DOI] [PubMed] [Google Scholar]
- Ishimoto A., Hartley J. W., Rowe W. P. Detection and quantitation of phenotypically mixed viruses: mixing of ecotropic and xenotropic murine leukemia viruses. Virology. 1977 Sep;81(2):263–269. doi: 10.1016/0042-6822(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Endogenous retroviruses. Cell. 1983 Jan;32(1):5–6. doi: 10.1016/0092-8674(83)90491-9. [DOI] [PubMed] [Google Scholar]
- Kozak C. A. Genetic mapping of a mouse chromosomal locus required for mink cell focus-forming virus replication. J Virol. 1983 Oct;48(1):300–303. doi: 10.1128/jvi.48.1.300-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Rowe W. P. Genetic mapping of ecotropic murine leukemia virus-inducing loci in six inbred strains. J Exp Med. 1982 Feb 1;155(2):524–534. doi: 10.1084/jem.155.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M., Sherr C., Potter M., Todaro G. Isolation of type-C viruses from the Asian feral mouse Mus musculus molossinus. Int J Cancer. 1975 Feb 15;15(2):211–220. doi: 10.1002/ijc.2910150206. [DOI] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Hartley J. W., Rowe W. P. Induction of B-tropic and N-tropic murine leukemia virus from B10.BR/SgLi mouse embryo cell lines by 5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1979 Jul;63(1):213–217. [PubMed] [Google Scholar]
- O'Brien S. J., Berman E. J., Estes J. D., Gardner M. B. Murine retroviral restriction genes Fv-4 and Akvr-1 are alleles of a single locus. J Virol. 1983 Sep;47(3):649–651. doi: 10.1128/jvi.47.3.649-651.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka T., Ikeda H., Moriwaki K., Matsuzawa A., Mizuno M., Kondo K. Genetic resistance in Japanese wild mice (Mus musculus molossinus) to an NB-tropic Friend murine leukemia virus. J Natl Cancer Inst. 1978 Nov;61(5):1301–1306. doi: 10.1093/jnci/61.5.1301. [DOI] [PubMed] [Google Scholar]
- Odaka T., Ikeda H., Yoshikura H., Moriwaki K., Suzuki S. Fv-4: gene controlling resistance to NB-tropic Friend murine leukemia virus. Distribution in wild mice, introduction into genetic background of BALB/c mice, and mapping of chromosomes. J Natl Cancer Inst. 1981 Nov;67(5):1123–1127. [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Senior A. M., Salazar F. H. Host Susceptibility to endogenous viruses: defective, glycoprotein-expressing proviruses interfere with infections. J Virol. 1981 Dec;40(3):745–751. doi: 10.1128/jvi.40.3.745-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. L., Bird S., Weinberg R. A. Evidence for the Asiatic origin of endogenous AKR-type murine leukemia proviruses. J Virol. 1980 Sep;35(3):824–835. doi: 10.1128/jvi.35.3.824-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S. FV-4: a new gene affecting the splenomegaly induction by Friend leukemia virus. Jpn J Exp Med. 1975 Dec;45(6):473–478. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Odaka T. Surface antigen expressed in hematopoietic cells derived from Fv-4r mouse strains. J Natl Cancer Inst. 1982 Jun;68(6):1005–1009. [PubMed] [Google Scholar]