Abstract
Recent reports have shown interplay between EETs (epoxides) and the heme oxygenase (HO) system in attenuating adipogenesis in cell culture models; prompting an examination of the effectiveness of EET agonist on obesity and associated cardio-metabolic dysfunction. Patho-physiological effects of an EET agonist (NUDSA) were contrasted in the absence and in the presence of stannous mesoporphyrin (an HO inhibitor) in SD rats fed a high fat (58%, HF) for 16 weeks. Animals on HF diet exhibited enhanced oxidative stress, increased levels of inflammatory cytokines and decreased levels of adiponectin along with reduced vascular and adipose tissue levels of EETs, HO-1; as compared to control rats (11% dietary fat). Treatment with NUDSA not only reversed serum adiponectin and vascular and adipose tissue levels of EETs and HO-1, but also, decreased blood pressure, subcutaneous and visceral fat content and serum TNFα and IL-6 levels in rats on HF diet. Aortic endothelial function, peNOS expression and adipose tissue markers of energy homeostasis i.e. pAMPK, Sirt1 and FAS, impaired in rats fed a HF diet, were restored in animals treated with this EET agonist. That NUDSA enhanced HO-1 expression, was accompanied by increase in p-GSK-3β and pAKT levels along with attenuation of adipose tissue levels of Bach 1 – the transcriptional suppresser of HO-1 expression. Prevention of these beneficial effects of NUDSA, in animals on HF diet and concurrently exposed to NUDSA and SnMP, supports the role of EET-HO interaction in mediating such effects. Taken together, our findings suggest that the EETs stimulate HO-1 expression via suppression of Bach 1 and interplay of these two systems affords vascular and metabolic protection in diet induced obesity.
Keywords: Cytochrome P450, HO-1, Metabolic dysfunction, Obesity, Adiponectin
1. Introduction
Epoxyeicosatrienoic acids (EETs) are catalyzed from arachidonic acid (AA) by a family of enzymes belonging to the Cytochrome P450 (CYP) super family [1,2]. Upon formation, EETs are rapidly hydrolyzed by soluble epoxide hydrolase (sEH) to their respective dihydroxyepoxytrienoic acids (DHETs) as well as to esterification products primarily to glycerophospholipids [3]. EETs exhibit potent biological effects including vasodilation, stimulation of ion transport, inhibition of inflammatory response and stimulation of epithelial cell growth [4–6]. Studies that demonstrate that the induction of CYP2C23 [7] and inhibition of sEH provide vascular protection [4,5] further highlight the role of EETs as modulators of vascular function. In addition, emerging studies indicate a role of this ubiquitous lipid mediator in the regulation of metabolic homeostasis [8]; thereby warranting further examination of its biological role in conditions such as obesity.
The heme oxygenase (HO) isoforms, HO-1 (inducible) and HO-2 (constitutive), gene expression is known to increase signaling molecules – mediated antioxidant and anti-inflammatory properties [9]. HO-1 is a stress response protein whose expression is principally regulated by transcription factors NRF2 and Bach 1. Where electrophiles activate NRF2-depndent HO-1 expression, molecules such as heme bind to and inhibit Bach 1 which is an inhibitor of HO-1 expression in quiescent cells. Increased HO-1 expression and activity is associated with an increase in adiponectin secretion and downstream signaling resulting in the stimulation of NO bioavailability [9–11]. HO-1 derived carbon monoxide (CO) regulates vascular tone in part by decreasing CYP450-derived vasoconstriction [12]. Furthermore, activation of the heme-HO system could contribute towards the vasodilatory effects of lipid mediators such as EETs [13,14].
Obesity, and associated metabolic syndrome, is a systemic inflection characterized by increased oxidative stress (ROS) along with inhibition of the HO-adiponectin system while increasing levels of inflammatory cytokines and insulin resistance [15,16]. We have recently reported that epoxides attenuate the metabolic syndrome like phenotype in the HO-2 null mice via the activation of HO-1 dependent pathways [8]. In addition, EETs attenuate adipocyte hypertrophy and improve adipocyte function in bone marrow derived mesenchymal stem cells (MSCs) via an increase in HO-1 expression [17]. In light of this evidence, the present study aims to explore the effectiveness of an EET agonist on the prevention of adiposity and a possible interaction between the HO and epoxide systems, in vivo, where metabolic pathologies have been induced in rats fed a HF diet. This study corroborates the existence of an epoxide-HO axis whose stimulation via an exogenous EET agonist (NUDSA) induces Bach 1-dependent HO-1 expression and abates obesity associated vascular dysfunction and improves metabolic homeostatic markers in visceral adipose tissues, in SD rats. Prevention of these beneficial effects in HF fed rats concurrently exposed to SnMP and NUDSA exposes interplay of epoxide-HO systems in affording aforementioned vasculo-metabolic protections.
2. Materials and methods
2.1. Materials
The EET analog, 11-(nonyloxy)undec-8(Z)-enoic acid (NUDA), is a potent vasorelaxant in mesenteric and renal arteries [18,19]. For in vivo studies, NUDA was conjugated with l-aspartic acid to form (S)-2-(11-(nonyloxy) undec-8(Z)-enamido)succinic acid (NUDSA) to minimize β-oxidation and improve solubility in aqueous milieu.
2.2. Animal experimentation
All animal experiments followed an institutionally approved protocol in accordance with the NIH Guidelines. Forty, 8-week-old Sprague-Dawley (SD) rats were used in the studies. Rats were divided into four groups (10 rats/group): (A) Control, (B) HF + vehicle, (C) HF + NUDSA, (D) HF + NUDSA + SnMP. Control rats (group A) were fed ad libitum a normal diet containing 11% fat, 62% carbohydrate, and 27.0% protein with a total calories of 12.6 kJ/g. The remaining animals (groups B, C, D) were fed a high-fat diet containing 58% fat (from lard), 25.6% carbohydrate, and 16.4% protein with total calories of 23.4 kJ/g (Bio-SERV, Frenchtown, NJ) for 16 weeks [20,21]. After 14 weeks of HF diet, NUDSA was injected, intraperitoneally, for 2 weeks daily at a dose of 1.5 mg/100 gm of body weight. Similarly after 14 weeks of HF diet, SnMP was injected intraperitoneally, 3 times a week at a dose of 20 mg/kg of body weight, for 2 weeks. After a 6-h fast, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and blood was obtained from a tail vein for glucose measurement using a glucometer (Lifescan Inc., Miligitas, CA). Blood pressure was measured by the tail cuff method immediately prior to NUDSA administration and every 7 days thereafter. Body weights of SD rats at the beginning of the experiment were 269 ± 12 g. At the time of sacrifice the body weight, visceral and subcutaneous fat content of all rats was measured. Blood samples were collected in K3EDTA tubes at sacrifice and the plasma was separated. Samples were flash frozen in liquid nitrogen and maintained at −80 °C until needed.
2.3. Assessment of vascular reactivity
The aorta was removed, cleaned of fat and loose connective tissue, placed in cold Krebs-bicarbonate solution, and sectioned into 3-mm-long rings. Vasorelaxation responses of phenylephrine-constricted arteries to cumulative increments in acetylcholine (10−9 to 10−4 mol/L) were examined in the presence of indomethacin (10 µmol/L) as described [8].
2.4. Measurement of EETs and DHETs
Aorta was homogenized in 66% methanol containing a 500 pg mixture of internal standards (PGE2-d4; 8(9)-EET-d11; 11(12)-EET-d8; 12-HETE-d8; 20-HETE-d6, and 11,12-DHET-d11). EETs concentration was calculated as described [8].
2.5. Western blot analysis of HO-1, Bach 1, GSK-3β, Sirt 1, FAS, pAKT, pAMPK, ferritin, eNOS, peNOS, adiponectin and phosphorylation of insulin receptor (IR)
Frozen tissues were used for determination of HO-1, Bach 1, GSK-3β (serine 9), Sirt 1, FAS, AMPK-alpha, pAMPK (Thr172), pAKT (serine 473), adiponectin, eNOS and peNOS (serine 1177), ferritin and insulin receptor phosphorylation. The nuclear fractions were obtained using Nuclear Extraction Reagents (Thermo Scientific, Rockford, IL, USA) and were done according to the manufacturer’s protocol. Immunoblotting was performed in aorta and adipose tissue as previously described [8,15].
2.6. O2− production
Aorta and adipose tissues were placed in scintillation vials respectively (2 per vial) containing 1 mL of Krebs-HEPES buffer, pH 7.4, and lucigenin (5 µmol/L) for 30 min at 37 °C. Lucigenin chemiluminescence was measured in a liquid scintillation counter (LS6000TA, Beckman Instruments) and superoxide production quantified as previously described [22].
2.7. Cytokines/chemokines and plasma insulin measurements
The levels of IL-6, TNF alpha and the high molecular weight (HMW) form of adiponectin were determined using an ELISA assay as previously described [8,15]. Insulin concentration in plasma was determined by ELISA (Linco Research, St. Charles, MO, USA) according to the manufactures’ protocol.
2.8. Statistical analysis
Data are expressed as means ± S.E.M. Significance of difference in mean values was determined using one-way analysis of variance followed by the Newman-Keul’s post hoc test. p < 0.05 was considered to be significant.
3. Results
3.1. Effect of a HF diet on body weight and fat content
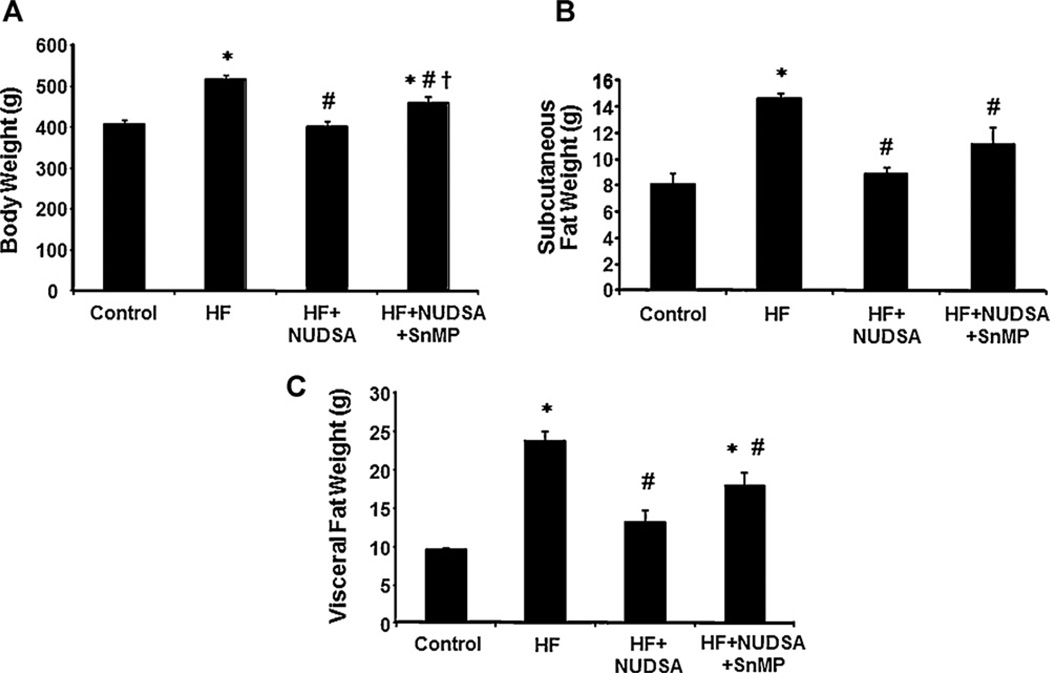
As seen in Fig. 1A, a HF diet for 16 weeks significantly increased body weight of SD rats as compared to age-matched controls (516 ± 9.4 vs. 407 ± 9.4 g, p < 0.01). This increase in body weight was attenuated in rats, on HF diet, treated with NUDSA for 2 weeks (p < 0.01). The decrease in body weight was reversed by the concurrent administration of SnMP and NUDSA to rats being fed a HF diet. These findings paralleled the change in subcutaneous fat content, Fig. 1B. Rats fed a HF diet showed marked increase in subcutaneous fat content as compared to control animals (14.7 ± 0.7 and 8.2 ± 1.6 g respectively, p < 0.05). Subcutaneous fat content was significantly (p < 0.05) decreased by NUDSA (9 ± 1.1 g) and this effect was prevented in animals exposed to SnMP and NUDSA. Similarly, visceral fat content was increased by HF diet and was reduced by NUDSA significantly as seen in Fig. 1C. Concurrent treatment with SnMP prevented NUDSA-induced attenuation of fat depots (p < 0.05) but not to the levels seen in animals fed a HF diet on vehicle treatment. These results indicate epoxide-mediated HO-dependent attenuation of body weight, along with reduction in subcutaneous and visceral fat depots, in animals on a HF diet.
Fig. 1.
Effect of a HF diet and treatment with NUDSA and NUDSA + SnMP in SD rats. Results are means ± SE, n = 10/group on (A) Body weight. *p < 0.01 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA. (B) Weight of subcutaneous fat. *p < 0.05 vs. control, #p < 0.05 vs. HF. (C) Weight of visceral fat. *p < 0.05 vs. control, #p < 0.05 vs. HF.
3.2. Effect of a HF diet on metabolic parameters
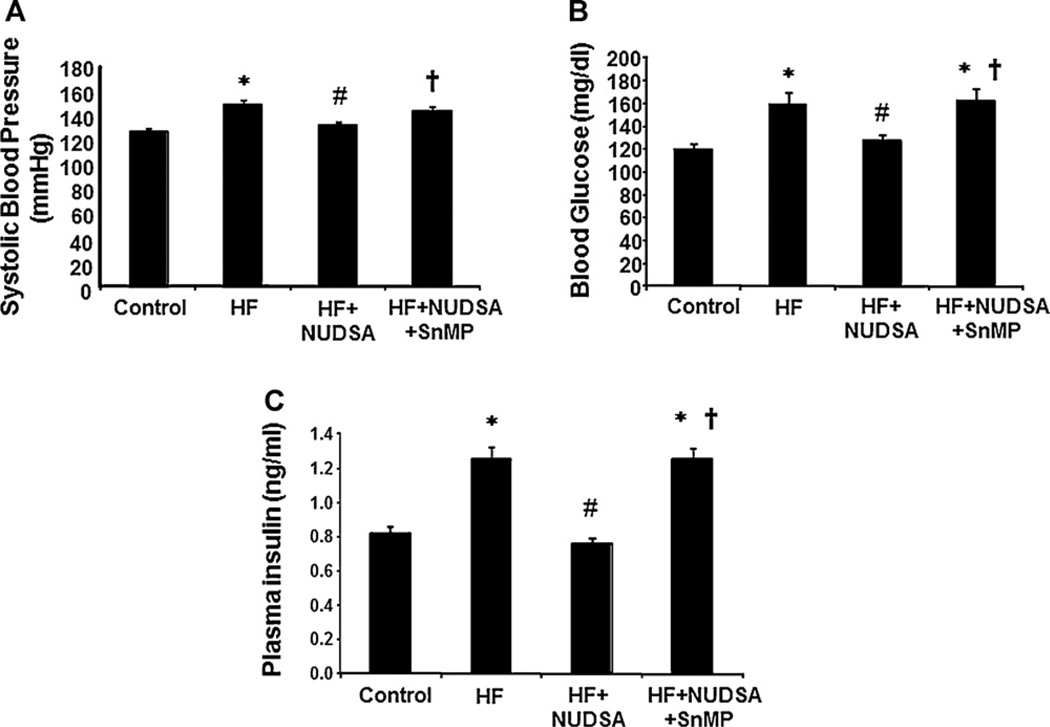
Systolic blood pressure was increased over the 16-week period of HF diet in SD rats (Fig. 2A; n = 10/group). The systolic blood pressure was 126.9 ± 2.6 mmHg in control animals and significantly increased in the rats fed a HF diet, 149.2 ± 4.1 mmHg (p < 0.01). This elevation in systolic pressure was attenuated (p < 0.01) by NUDSA treatment in animals fed a HF diet whereas SnMP nullified this antihypertensive effect of NUDSA (Fig. 2A). As expected, plasma glucose levels in rats fed a HF diet were higher than those in control animals (159 ± 19.4 and 119 ± 6.9 mg/dl respectively, p < 0.05) (Fig. 2B). This increase in blood glucose levels was also attenuated (128 ± 9.1, p < 0.05) by NUDSA treatment in rats fed a HF diet; an effect reversed by SnMP (162 ± 19.3 mg/dl, p < 0.05). Similar pattern was observed in plasma insulin levels (Fig. 2C). Rats fed a HF diet showed increased levels of plasma insulin (1.36 ± 0.08 ng/ml, p < 0.01) as compared to the control animals and this increase in insulin levels was also attenuated by NUDSA treatment in rats fed a HF diet; an effect reversed by SnMP (p < 0.05).
Fig. 2.
Effect of a HF diet and treatment with NUDSA and NUDSA + SnMP in SD rats. Results are means ± SE, n = 10/group on (A) Blood pressure, which was measured by tail cuff method. *p < 0.01 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA. (B) Blood glucose in samples obtained after a 6 h fast. *p < 0.05 vs. control, #p < 0.05 vs. HF, †p < 0.05 vs. HF + NUDSA. (C) Plasma insulin levels in samples obtained after a 6 h fast. *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA.
3.3. Effect of a HF diet on inflammatory cytokines and plasma adiponectin levels
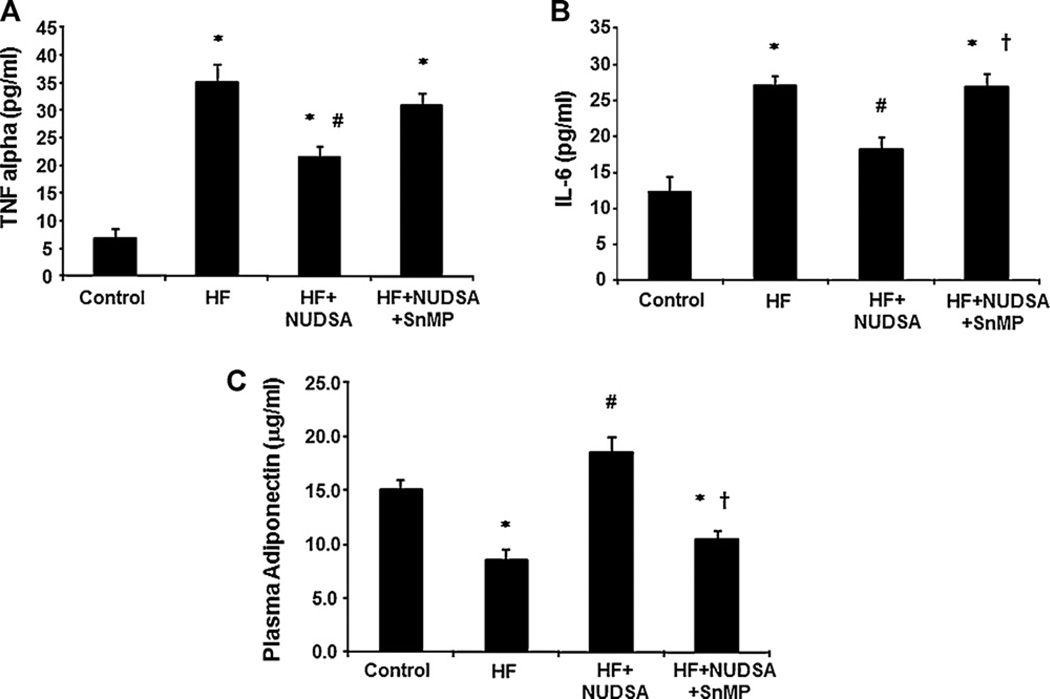
Diet-induced obesity is frequently accompanied by systemic inflammation and this was examined in the current study by assessment of circulating pro and anti-inflammatory markers. Plasma TNFα and IL-6 levels were greater in rats fed a HF diet compared to rats on a normal diet, Fig. 3A and B, (p < 0.05). NUDSA (p < 0.01) decreased plasma TNFα and IL-6 levels and this effect was also prevented by concurrent SnMP treatment (p < 0.01, Fig. 3A and B). Contrary to the patterns observed above, plasma adiponectin levels were lower in rats fed a HF diet when compared to control animals (p < 0.05; n = 10/group) (Fig. 3C). Plasma adiponectin levels were increased in rats on HF diet and treated with NUDSA (p < 0.01). Co-treatment with SnMP and NUDSA, in the rats fed a HF diet, prevented the increase in adiponectin levels observed with NUDSA alone.
Fig. 3.
Effect of a HF diet and treatment with NUDSA and NUDSA + SnMP in SD rats on plasma cytokines and adiponectin levels. Results are means ± SE, n = 10/group. (A) Plasma TNF alpha; *p < 0.05 vs. control, #p < 0.01 vs. HF. (B) Plasma IL-6; *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.01 vs. HF + NUDSA. (C) Plasma adiponectin levels. n = 10; *p < 0.01 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA.
3.4. Effect of a HF diet on vascular function
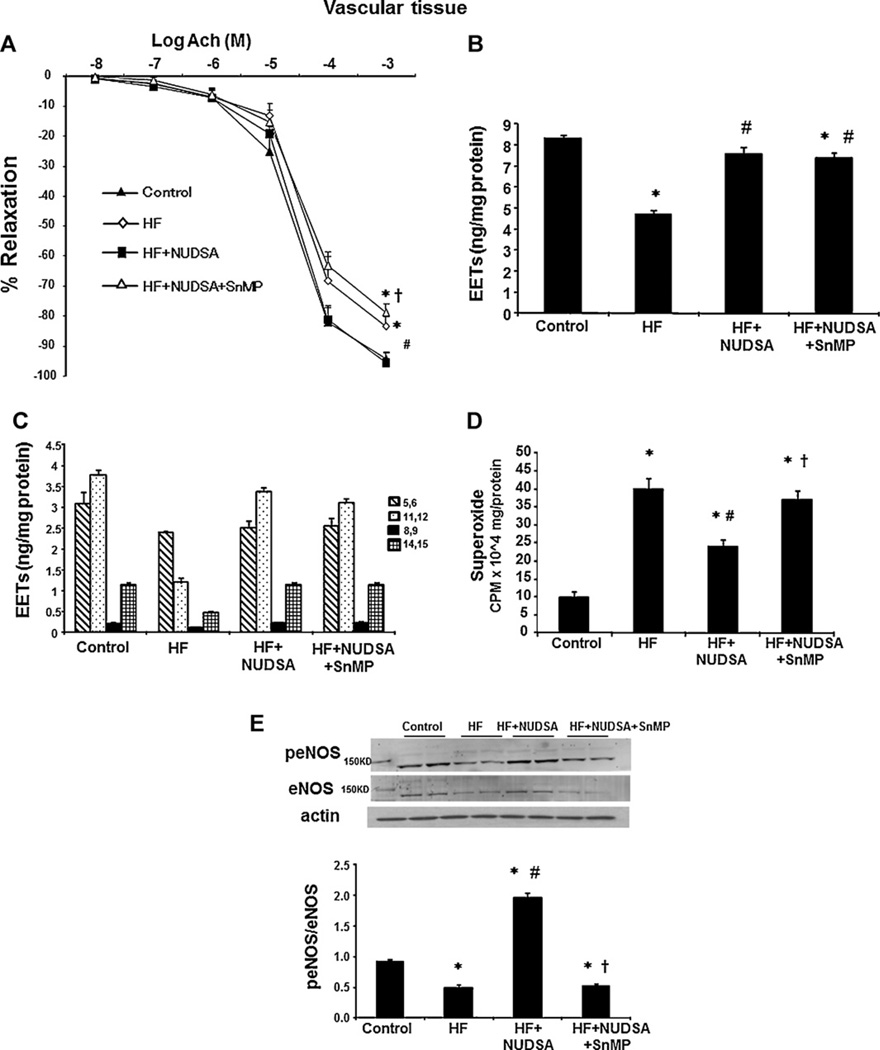
We studied the vascular parameters including acetylcholine-induced relaxation, O2− levels, EET levels and p-eNOS expression in rats fed a HF diet. Vascular function, measured as the relaxation in response to acetylcholine, was impaired in rats fed a HF diet, although, the statistical significance (p < 0.05) was only achieved at maximal dose of acetylcholine. As seen in Fig. 4A, at 10−3 M acetylcholine, the percent of relaxation was 83.27 ± 3.01% in rats fed a HF diet (p < 0.05) vs. 94.22 ± 2.03% in control animals. Treatment with NUDSA restored maximal relaxation to acetylcholine to levels no different from those recorded in animals fed control diet. Relaxation to 10−3 M acetylcholine increased (p < 0.05) by treatment with NUDSA from 83.27 ± 3.01 to 95.35 ± 3.34% in SD rats on HF diet (Fig. 4A) and this effect was reversed by SnMP (maximal dilation − 78.55 ± 2.55% vs. HF + NUDSA, p < 0.05).
Fig. 4.
Effect of NUDSA and NUDSA + SnMP on vascular parameters in SD rats fed a HF diet. (A) Relaxation-response curves to acetylcholine in femoral artery. Results are means ± SE, n = 5; *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA. (B) Total EETs levels in arteries. Results are means ± SE, n = 6; *p < 0.01 vs. control, #p < 0.05 vs. HF, †p < 0.05 vs. HF + NUDSA. (C) EET regioisomers in arteries. (D) Superoxide levels in aorta measured in SD rats. Results are means ± SE, n = 6; *p < 0.01 vs. control, #p < 0.05 vs. HF, †p < 0.05 vs. HF + NUDSA. (E) Western blot and densitometry analysis peNOS and eNOS proteins in aorta. Data are shown as mean band density normalized to β-actin, Results are means ± SE, n = 6, *p < 0.01 vs. control, #p < 0.01 vs. HF, †p < 0.01 vs. HF + NUDSA.
The impaired relaxation of acetylcholine was associated with a marked decrease in the EET levels in rats fed a HF diet (Fig. 4B and C). All the four EET regioisomers 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET were measured as shown in Fig. 4C. Total EET levels were 50% lower in HF diet rats as compared to control rats and amounted to 4.17 ± 0.04 ng/mg as compared to 8.36 ± 0.13 ng/mg respectively (p < 0.01). As seen in Fig. 4B, treatment with NUDSA restored aortic EETs levels to control levels in rats fed a HF diet, (p < 0.01).
As seen in Fig. 4D, vascular oxidative stress was increased as superoxide generation was greater in rats fed a HF diet compared to rats fed a normal diet (p < 0.05). NUDSA administration prevented the increase in aortic O2− generation in rats fed a HF diet (p < 0.05), an effect abolished by SnMP.
A HF diet was also associated with significant (p < 0.01) decrease in the aortic expression of peNOS/eNOS levels when compared to those in control animals (Fig. 4E). Treatment with NUDSA increased these levels by 2.5–3.0-fold to levels significantly (p < 0.01) higher than those in HF diet animals. This effect was significantly (p < 0.05) decreased by concurrent administration of SnMP.
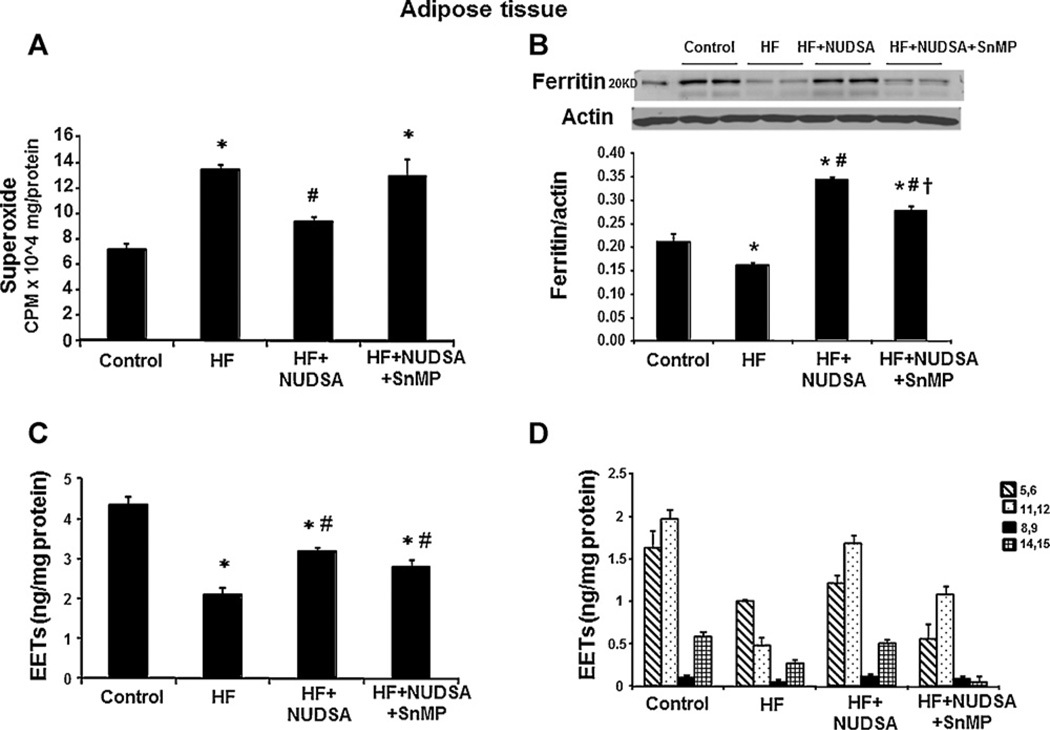
3.5. Effect of HF diet on oxidative stress, ferritin expression and EETs levels in visceral adipose tissues
Oxidative stress was assessed in adipose tissues by measurement of O2− levels, which was increased in rats fed a HF diet as compared to rats on a normal diet (p < 0.05), Fig. 5A. Treatment with NUDSA not only attenuated O2− generation, in rats fed a HF diet (p < 0.05), but also restored depressed ferritin levels (Fig. 5B); a cellular marker of redox balance. These effects of NUDSA were abolished in animals on HF diet concurrently exposed to SnMP. Adipose tissue levels of total EETs and the regioisomers were the reverse image of superoxide as seen in Fig. 5C and D. EET levels were lower in HF diet rats as compared to control rats and amounted to 2.0 ± 0.06 ng/mg as compared to 4.3 ± 0.3 ng/mg respectively (p < 0.01). Treatment with NUDSA increased EETs levels in rats fed a HF diet, (p < 0.01) and this effect was reversed by SnMP.
Fig. 5.
Effect of a HF diet and treatment with NUDSA and NUDSA + SnMP in SD rats on metabolic parameters in visceral adipose tissue. Results are means ± SE, n = 6/group. (A) Superoxide levels in visceral adipose tissues. *p < 0.05 vs. control, #p < 0.05 vs. HF. (B) Western blot and densitometry analysis of ferritin expression. Data are shown as mean band density normalized to β-actin, *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.01 vs. HF + NUDSA. (C) EETs levels in adipose tissues. *p < 0.01 vs. control, #p < 0.05 vs. HF. (D) EETs regioisomers in adipose tissues.
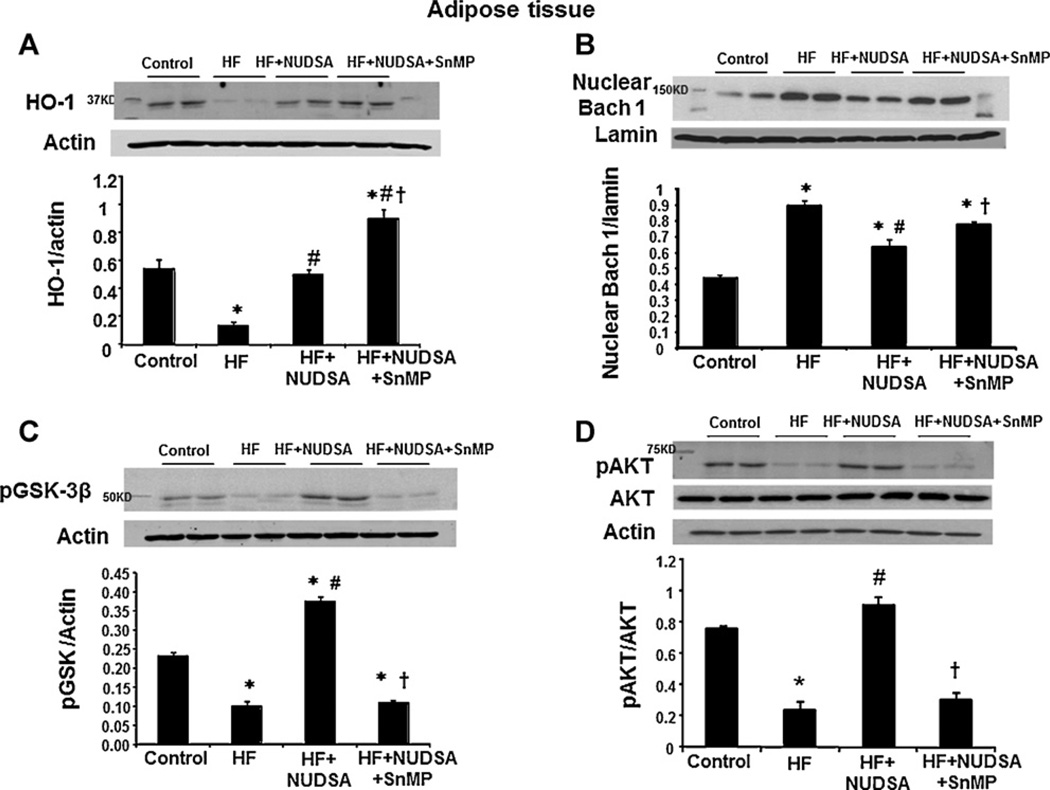
3.6. Effect of HF diet on HO-1, Bach 1, GSK-3β and p-AKT levels in visceral adipose tissues
We evaluated the proposed interplay between epoxides and HO-adiponectin systems in this, diet-induced, model of metabolic syndrome. Western blot analysis showed a significant decrease HO-1 expression (p < 0.01) in the visceral adipose tissues of rats fed HF diet as compared to rats on a control diet (Fig. 6A). NUDSA treatment increased HO-1 protein levels in animals treated with HF diet, which was further increased in animals exposed to SnMP. These findings are not surprising as SnMP, which induced a significant increase in HO-1 expression (Fig. 6A), is a potent inhibitor of HO activity, as shown previously [23], and thus prevents heme degradation and inhibits formation of HO products i.e. CO and biliverdin. Epoxide analog, NUDSA, also reduced (p < 0.05) nuclear levels of the electrophile-independent HO regulator, Bach 1 (Fig. 6B). This effect of NUDSA was associated with increased levels of p-AKT (Fig. 6D) and enhanced phosphorylation of Bach 1 regulator GSK-3β on a serine residue # 9 (Fig. 6C). Co-treatment with NUDSA and SnMP attenuated (p < 0.05) p-Akt and p-GSK-3β levels accompanied by restoration of nuclear levels of Bach 1.
Fig. 6.
Effect of a HF diet and treatment with NUDSA and NUDSA + SnMP in SD rats on the expression of HO-1, Bach 1, GSK-3β and pAKT in visceral adipose tissue. Data are shown as mean band density normalized to lamin and β-actin respectively, Results are means ± SE, n = 6; (A) Western blot and densitometry analysis of HO-1 expression. Data are shown as mean band density normalized to β-actin, *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.01 vs. HF + NUDSA. (B) Western blot and densitometry analysis of nuclear Bach 1 protein. *p < 0.01 vs. control, #p < 0.05 vs. HF, †p < 0.05 vs. HF + NUDSA. (C) Western blot and densitometry analysis of cytosolic GSK-3β. *p < 0.05 vs. control, #p < 0.05 vs. HF, †p < 0.05 vs. HF + NUDSA. (D) Western blot and densitometry analysis of pAKT in cytosol. *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA.
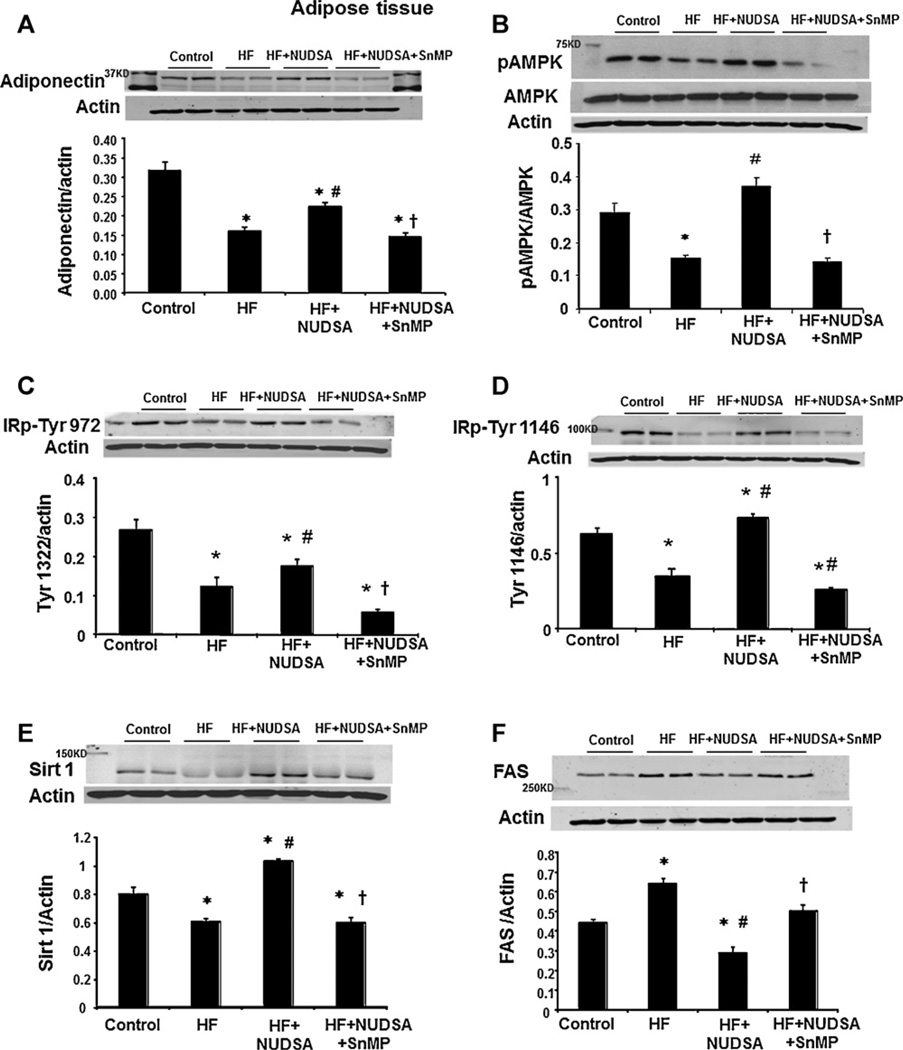
3.7. Effect of HF diet on molecular regulators and markers of metabolic homeostasis in visceral adipose tissues
Activation of the heme-HO system has been linked to the downstream activation and release of the protective adipokine, adiponectin. Western blot analysis of adiponectin levels, normalized against actin, showed a significant reduction (p < 0.01) in rats fed a HF diet compared to control animals, Fig. 7A. NUDSA administration resulted in increased (p < 0.05) expression of adiponectin levels and this effect was reversed by SnMP. A similar pattern was observed in pAMPK protein expression, Fig. 7B. HF diet decreased pAMPK/AMPK levels as compared to rats fed a normal diet (p < 0.05). NUDSA administration resulted in increased (p < 0.05) phosphorylation of AMPK and this effect was reversed by SnMP.
Fig. 7.
Effect of a HF diet and treatment with NUDSA and NUDSA + SnMP in SD rats on the expression of adiponectin, pAMPK, IR phosphorylation, sirt-1 and FAS in visceral tissue. Data are shown as mean band density normalized to β-actin. Results are means ± SE, n = 6. (A) Western blot and densitometry analysis of adiponectin proteins. *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.01 vs. HF + NUDSA. (B) Western blot and densitometry analysis of pAMPK and AMPK proteins. *p < 0.05 vs. control, #p < 0.05 vs. HF, †p < 0.01 vs. HF + NUDSA. (C and D) Western blot and densitometry analysis of insulin receptor phosphorylation, P-Tyr 972 and Tyr-1146 respectively. *p < 0.05 vs. control, #p < 0.05 vs. HF, †p < 0.01 vs. HF + NUDSA. (E) Western blot and densitometry analysis of cytosolic Sirt1. *p < 0.05 vs. control, #p < 0.05 vs. HF, †p < 0.05 vs. HF + NUDSA. (F) Western blot and densitometry analysis of FAS in cytosol. *p < 0.05 vs. control, #p < 0.01 vs. HF, †p < 0.05 vs. HF + NUDSA.
We next examined the effect of NUDSA treatment on insulin receptor phosphorylation in rats fed a HF diet. Phosphorylation of insulin receptors at sites Tyr-972 and Tyr-1146 was examined and shown in Fig. 7C and D; insulin phosphorylation at sites 972 and 1146 was significantly (p < 0.05) decreased in rats fed a HF diet as compared to rats on control diet. Densitometry analysis showed that NUDSA treatment increased the expression of P-Tyr 972 and Tyr-1146 and this effect was reversed by SnMP as shown in Fig. 7C and D.
Sirtuin 1 levels in cytosol showed a decreased expression in animals fed a HF diet as compared to control animals, Fig. 7E (p < 0.05). NUDSA administration resulted in increased (p < 0.05) expression of Sirt 1 levels and this effect was reversed by SnMP. We next examined the effect of NUDSA treatment on FAS in rats fed a HF diet as shown in Fig. 7F. FAS levels showed a significant increase (p < 0.01) in rats fed a HF diet compared to control animals, Fig. 7F. NUDSA administration resulted in decreased (p < 0.05) expression of FAS levels and this effect was reversed by SnMP (Fig. 8).
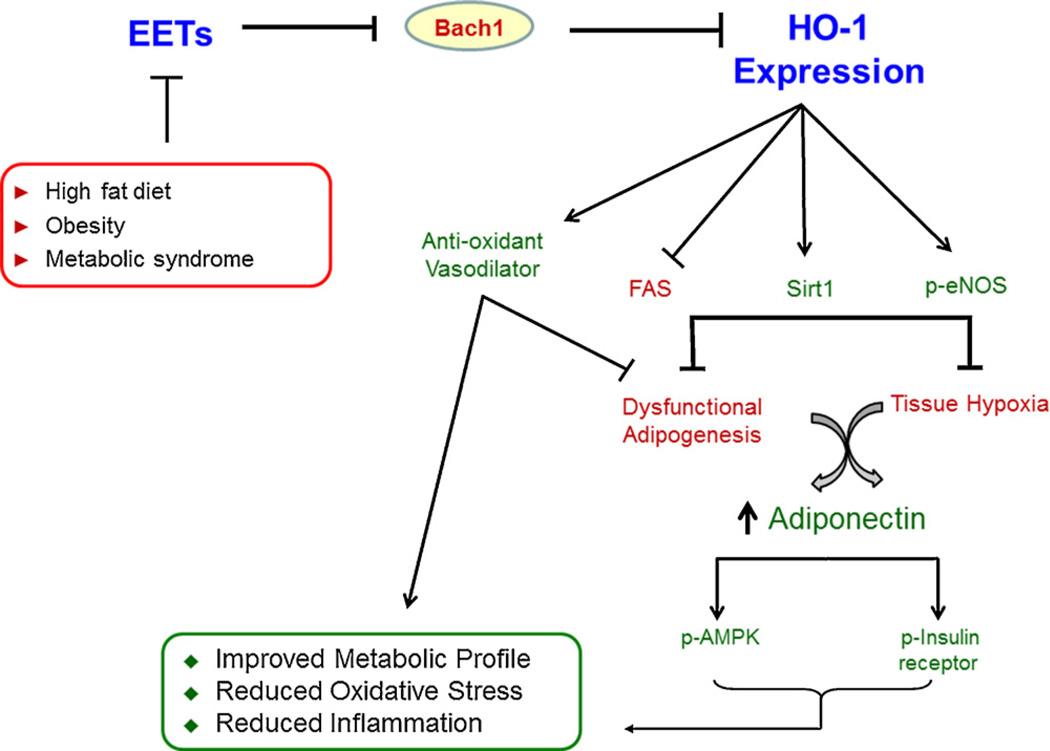
Fig. 8.
Proposed mechanisms involved in epoxide-mediated HO-1-induction and their downstream effects. HF diet in conjunction with chronic oxidative stress inhibits epoxide synthesis and attenuates heme-HO-adiponectin axis. Administration of an EET analog, NUDSA, induces Bach 1-dependent HO-1 expression that in turn contributes towards abrogation of metabolic patho-physiological alterations, partly via stimulation of adiponectin dependent pathways including p-AMPK and insulin receptor phosphorylation.
4. Discussion
This study demonstrates an inter-dependent relationship between CYP 450 derived epoxides and the heme-HO system in mediating the regulation of metabolic balance. Our results clearly establish the development of vascular and adipose tissue dysfunction in SD rats fed a HF diet, which is amenable to rescue by the EET agonist, NUDSA. In addition, the prevention of these beneficial effects of NUDSA by heme-HO inhibitor strongly implicates the existence of a synergistic relationship between these systems as a positive regulator of metabolic homeostasis.
The first key finding reported here is an attenuative effect of HF diet-induced obesity on vascular and adipose tissue levels of epoxides. In accordance with earlier studies [24], HF diet led to hyperglycemia, insulin resistance, vascular dysfunction and hypertension in SD rats. Also, HF diet has been linked to chronic inflammation and enhanced levels of oxidative stress [24–26]. The results described here corroborate these findings in that both vasculature and visceral adipose tissue. Both exhibited a marked increase in oxidative stress along with a significant increase in markers of systemic inflammation. These alterations in cellular redox are accompanied by the decrease in EETs, HO-1 and adiponectin levels. The inability of rats fed a HF diet to sustain normal levels of EET may be the result of the increased levels of ROS which have been shown to affect EET bioavailability [27–30]. Also, we show here that increased levels of the ROS accelerate the degradation of the intracellular iron binding protein ferritin [31]. This cellular effect of ROS may result in increased cytoplasmic pools of free iron thus facilitating the Fenton reaction [9]. The Fenton’s reaction generates hydroxyl radical (OH−), a strong oxidizing agent, further exacerbating cellular redox imbalance. This oxidative stress may also contribute towards reduced epoxide levels via increased expression of COX-2, thus, diverting the common substrate i.e. arachidonic acid towards cyclooxygenase instead of epoxygenases pathway. The reversal of these detrimental biochemical processes by NUDSA involves the activation of the heme-HO pathway and the generation of the heme degradation products CO and biliverdin, the latter a powerful antioxidant [32]. Increased HO-1 levels and the concomitant decrease in levels of ROS observed with NUDSA may prevent EET degradation, as suggested by reversal of tissue levels of ferritin and epoxides.
The second key finding observed in this study is an interplay between epoxides and the heme-HO system, where beneficial effects of NUDSA in vascular and adipose tissues is prevented in animals exposed to an inhibitor of HO activity. The heme-HO system is a stress response system (reviewed in [33]) that undergoes activation under conditions of increased oxidative stress, such as those presented here. However, in agreement with our earlier report [22], present findings support an insufficient stimulation of this enzyme system under conditions exhibiting chronic oxidative stress. The animals fed a HF diet showed lower levels of HO-1 as compared to animals fed a normal diet. Although the precise mechanisms involved remain unclear, an inhibitory effect of chronic oxidative stress on heme-oxygenase activity [34] has also been reported by other investigators. In addition, upregulation of HO-1 may be, in part, reliant upon CYP450 derived epoxides [13,14] whose subnormal levels in rats fed a HF diet could contribute towards inappropriate activation of HO-1.
One of the possible mechanisms via which epoxides enhance HO-1 expression may involve the transcriptional regulator Bach 1. Bach 1 is a constitutively active HO-1 suppressor binding to the antioxidant response elements (AREs) in the HO-1 gene [35]. Bach 1 suppresses HO-1 transcription and its inhibition has been reliantly shown to induce HO-1 [36–38]. We show here for the first time that an epoxide agonist can attenuate Bach 1 and disinhibit HO-1 expression, in an in vivo setting. Bach 1 in turn has been postulated to be regulated by GSK-3β [35], which itself is a substrate for PI3/AKT pathway [39,40]. In agreement with studies from our lab and other investigators [17,41], we show here an EET-mediated increase in p-AKT levels which in turn are accompanied by increased phosphorylation of GSK-3β; this phosphorylation of GSK-3β been documented to attenuate it’s enzymatic activity. Increase in GSK-3β phosphorylation, in our studies, is mirrored by attenuated levels of nuclear Bach 1 which may lead to reduced binding of Bach 1 to the AREs of HO-1 gene. These interactions transpire increased transcription and expression of HO1, as observed in NUDSA treated rats. Thus, EET-dependent HO-1 upregulation may involve inhibition of GSK-3β with downstream regulation of Bach 1. Further studies are required, however, to establish a direct linkage between constitutively active GSK-3β and Bach 1s nuclear localization which may involve a yet unidentified phosphorylation site on the latter.
Role of p-AKT/GSK-3β in regulating nuclear Bach 1 is further supported by SnMP-dependent attenuation of p-AKT and p-GSK-3β levels accompanied by elevation of nuclear Bach 1. HO-adiponectin axis has been documented to increase p-AKT levels in a variety of tissues and attenuative effects of SnMP on the p-AKT/GSK-3β pathway could be a result of its inhibitory effects on this axis. However, SnMP, which inhibits HO activity, also induces HO-1 expression without attenuation of Bach 1. This contradictory effect of SnMP cannot be fully explained at this time but may represent a direct stimulatory or an alternative pathway (Bach 1 independent) of metallo-porphyrin-mediated HO-1 induction [42].
Reduced HO-1 levels and HO activity are accompanied by a reduction in the HO-derived metabolites biliverdin and CO and their signaling capacity in vascular protection and cell function (Reviewed in [43,44]). This is confirmed in the present study by a marked decrease in adiponectin, pAMPK and IR phosphorylation in adipose tissue. Although, precise molecular mechanisms involved in HO-1-mediated adiponectin release are not fully understood, a few possible mechanisms may be involved: firstly, in vitro studies have established HO-1-mediated attenuation of Adipocyte hypertrophy which in turn reduces dysfunctional adipogenesis and could promote adiponectin synthesis and release [17,45]. Secondly, HO-1 induction has been linked to reduced inflammatory response and improved tissue perfusion [9], both of which have the potential to increase adiponectin synthesis and release from adipose tissues. Hence, epoxide-induced, increased HO-1 expression can reduce hypertrophic adipogenesis and abate systemic inflammatory response, both of which could mediate enhanced adiponectin release. Activation of AMPK is critical in cellular energy homeostasis through the stimulation of glucose transport, the switching off of energy consumption by decreasing lipogenesis, the increase in fatty acid oxidation and ATP levels [46,47]. Increased phosphorylation of insulin receptors in adipose tissue may be a response to the increase in pAMPK and pAKT pathways indicating improved glucose tolerance [22]. A synergistic interplay of these physiological mechanisms could contribute towards improvement of metabolic homeostasis observed in NUDSA treated animals.
Apart from HO-1, adiponectin and pAMPK-mediated improvement in energy balance, EET agonist-induced attenuation of subcutaneous and visceral adiposity could also involve an increase in cellular levels of Sirt1 and a reduction in FAS. Sirt1 is a histone deacytelase, which attenuates adipogenesis [48] via inhibition of PPARγ- the master regulator of adipogenesis [49]. We have already shown inhibitory effects of EETs on adipogenesis along with inhibition of PPARγ expression in in vitro experiments [17]. Furthermore, NUDSA treatment attenuated FAS, an enzyme critical for fat synthesis in adipose tissues. SnMP-mediated reversal of these effects implicates EET agonist-mediated induction of HO-1 as the determinant of these effects of NUDSA.
That vascular endothelial dysfunction and elevated blood pressure, observed in rats on HF diet, was prevented in animals concurrently exposed to EET agonist is also a reflection of improved physiological homeostasis in these animals. Such effects could involve increased eNOS activity, as evidenced by enhanced eNOS phosphorylation, and/or increased NO bioavailability attributable to an antioxidant milieu afforded by increased HO-1 expression. In addition, CO, a product of heme catabolism by HO and epoxides themselves are direct vasodilators [50,51] and can contribute towards improved vascular function observed in NUDSA treated animals. Improved vascular function and reduced oxidative stress could also contribute towards reduced adiposity and reduced adipose tissue dysfunction via abatement of cellular hypoxia [52].
In addition, increase in adiponectin, observed in NUDSA treated rats, could facilitate eNOS activation via crosstalk between pAKT and pAMPK [53,54]. An increase in AMPK-AKT signaling is considered an important metabolic response key to the attenuation of ROS-mediated endothelial dysfunction, since pAMPK and pAKT use eNOS as a substrate and enhance the levels of peNOS (44). This suggests that the EET-HO-1 mediated prevention of vascular and adipose tissue dysfunction is related to increases in adiponectin-pAMPK signaling pathways [55], the trigger for the phenotype change and resistance to oxidative stress.
5. Conclusion
In conclusion, as represented in the schematic, the present data suggests that HF diet in conjunction with chronic oxidative stress inhibits epoxide synthesis and attenuates heme-HO-adiponectin axis. These effects are reflected downstream as increased adiposity, vascular endothelial dysfunction, systemic inflammatory response and altered metabolic homeostasis. Administration of an EET analog, NUDSA, induces Bach 1-dependent HO-1 expression that in turn contributes towards abrogation of above-mentioned pathophysiological alterations, partly via stimulation of adiponectin dependent pathways including p-AMPK and insulin receptor phosphorylation. Where, the present study examines and advances the field of diet induced obesity and metabolic syndrome, it does not identify precise molecular mechanisms involved in some of the beneficial attributes of the heme-HO system, including its activation of the adiponectin axis. Future studies in such matters could provide crucial insights into the complex molecular mechanisms, and their physiological consequences, involved in the regulation of metabolic homeostasis. However, identification of the inhibitory effect of obesity on the epoxide-HO pathways responsive to an EET analog does open fresh avenues for pharmaco-therapeutic interventions aimed towards treatment of such disorders.
Acknowledgments
This work was supported by NIH grants DK068134, HL55601 and HL34300 (NGA). All authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written. We thank Jennifer Brown for her outstanding editorial assistance in the preparation of the manuscript.
Abbreviations
- CYP
cytochrome P450
- EETs
epoxyeicosatrienoic acids
- NUDSA
EET agonist
- HO
heme oxygenase
- SnMP
stannous mesoporphyrin
- ROS
reactive oxygen species
- IR
insulin receptor
- O2−
superoxide
- sEH
soluble epoxide hydrolase
References
- 1.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 2.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 3.Karara A, Dishman E, Falck JR, Capdevila JH. Endogenous epoxyeicosatrienoyl-phospholipids. A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J Biol Chem. 1991;266:7561–7569. [PubMed] [Google Scholar]
- 4.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 5.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 6.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 7.Muller DN, Theuer J, Shagdarsuren E, et al. A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol. 2004;164:521–532. doi: 10.1016/s0002-9440(10)63142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sodhi K, Inoue K, Gotlinger K, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 10.Kruger AL, Peterson SJ, Schwartzman ML, et al. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 11.L’Abbate A, Neglia D, Vecoli C, et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H3532–H3541. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]
- 12.Kaide J-I, Zhang F, Wei Y, et al. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J Clin Invest. 2001;107:1163–1171. doi: 10.1172/JCI11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacerdoti D, Bolognesi M, Di PM, et al. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 14.Sacerdoti D, Colombrita C, Di PM, et al. 11,12-epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;82:155–161. doi: 10.1016/j.prostaglandins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Kim DH, Tsenovoy PL, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 16.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Vanella L, Inoue K, et al. EET-agonist regulates human mesenchymal stem cells-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19(12):1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitropoulou C, West L, Field MB, et al. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998;136:17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 21.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 22.Peterson SJ, Kim DH, Li M, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botros FT, Schwartzman ML, Stier CT, Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005;68:2745–2755. doi: 10.1111/j.1523-1755.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 24.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high fat diet hypertensive rats. Clin Sci (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight SF, Yuan J, Roy S, Imig JD. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am J Physiol Renal Physiol. 2010;298:F86–F94. doi: 10.1152/ajprenal.00351.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen BT, Gutterman DD, Sato A, et al. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol. 2000;279:F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 29.Wang MH, Smith A, Zhou Y, et al. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–599. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- 30.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Malaguarnera L, Quan S, Pilastro MR, Abraham NG, Kappas A. Diminished heme oxygenase potentiates cell death: pyrrolidinedithiocarbamate mediates oxidative stress. Exp Biol Med (Maywood) 2003;228:459–465. doi: 10.1177/15353702-0322805-06. [DOI] [PubMed] [Google Scholar]
- 32.Abraham NG, Drummond G. CD163-Mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ Res. 2006;99:911–914. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- 33.Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol. 2009;297:F1137–F1152. doi: 10.1152/ajprenal.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinobe R, Ji Y, Nakatsu K. Peroxynitrite-mediated inactivation of heme oxygenases. BMC Pharmacol. 2004;4:26. doi: 10.1186/1471-2210-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Vanella L, Kim DH, Sodhi K, et al. Crosstalk between EET and HO-1 down-regulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96:54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamuro T, Takahashi K, Ogawa K, et al. Bach 1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 38.Reichard JF, Sartor MA, Puga A. BACH1 is a specific repressor of HMOX1 that is inactivated by arsenite. J Biol Chem. 2008;283:22363–22370. doi: 10.1074/jbc.M801784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin S, Chock PB. Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt. Biochemistry (Mosc) 2003;42:2995–3003. doi: 10.1021/bi0205911. [DOI] [PubMed] [Google Scholar]
- 40.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhary KR, Batchu SN, Das D, et al. Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated improved post-ischaemic recovery of heart contractile function. Cardiovasc Res. 2009;83:362–370. doi: 10.1093/cvr/cvp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cable EE, Gildemeister OS, Pepe JA, Lambrecht RW, Bonkovsky HL. Mechanism of induction of heme oxygenase by metalloporphyrins in primary chick embryo liver cells: evidence against a stress-mediated response. Mol Cell Biochem. 1997;169:13–20. doi: 10.1023/a:1006817207166. [DOI] [PubMed] [Google Scholar]
- 43.Stec DE, Drummond HA, Vera T. Role of carbon monoxide in blood pressure regulation. Hypertension. 2008;51:597–604. doi: 10.1161/HYPERTENSIONAHA.107.097154. [DOI] [PubMed] [Google Scholar]
- 44.Kravets A, Hu Z, Miralem T, Torno MD, Maines MD. Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J Biol Chem. 2004;279:19916–19923. doi: 10.1074/jbc.M314251200. [DOI] [PubMed] [Google Scholar]
- 45.Kim DH, Burgess AP, Li M, et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 46.Tomas E, Tsao TS, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laderoute KR, Amin K, Calaoagan JM, et al. 5’-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawai M, Rosen CJ. PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaide J, Zhang F, Wei Y, et al. Vascular CO counterbalances the sensitizing influence of 20-HETE on agonist-induced vasoconstriction. Hypertension. 2004;44:210–216. doi: 10.1161/01.HYP.0000135658.57547.bb. [DOI] [PubMed] [Google Scholar]
- 51.Larsen BT, Miura H, Hatoum OA, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 53.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 54.Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambuceti G, Morbelli S, Vanella L, et al. Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage; reversed by increases in pAMPK, heme oxygenase-1 and adiponectin. Stem Cells. 2009;27:399–407. doi: 10.1634/stemcells.2008-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]