Abstract
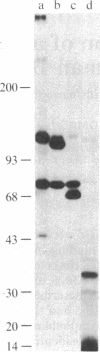
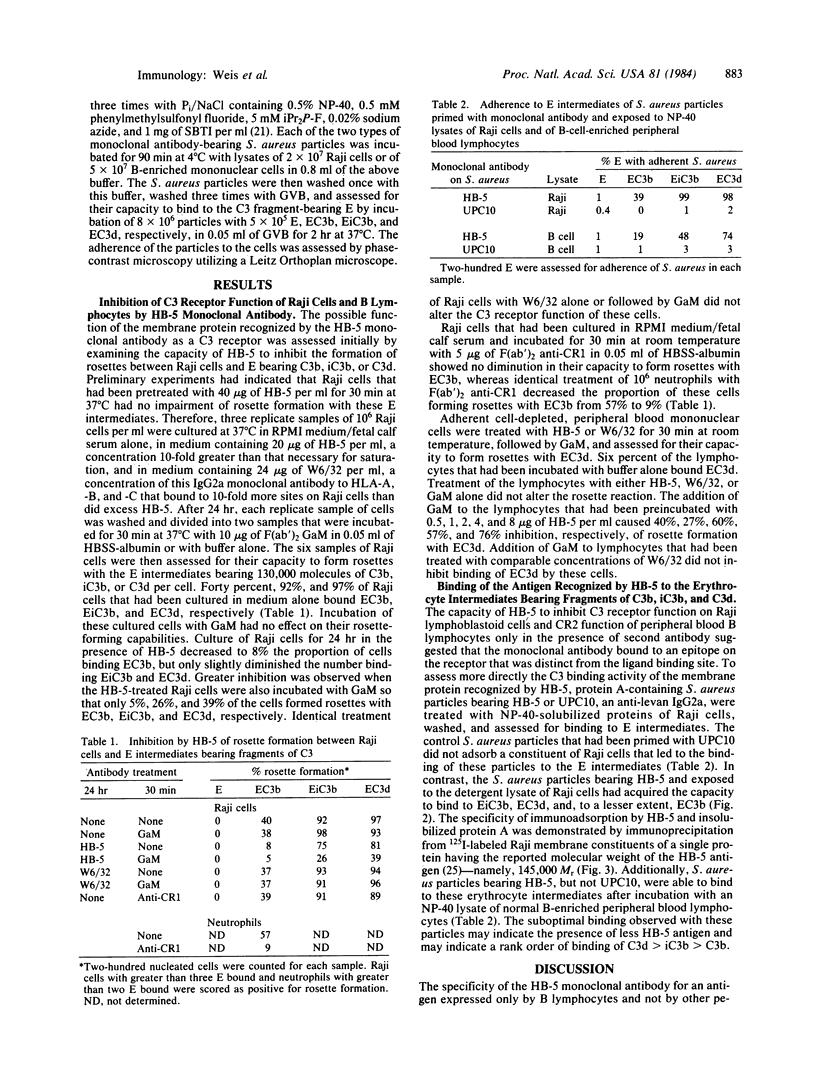
The C3d receptor (CR2) of human B lymphocytes mediates the binding to these cells of immune complexes that have activated the complement system and bear the fragments of C3, iC3b, C3d,g, and C3d. A 145,000 Mr membrane protein previously described as being recognized by the monoclonal antibody HB-5 and shown to be expressed only by B lymphocytes and B lymphoblastoid cell lines, such as Raji, was assessed for its possible identity as CR2. Treatment of Raji cells with HB-5 and goat F(ab')2 anti-mouse IgG (GaM) diminished the capacity of these cells to form rosettes with sheep erythrocyte (E) intermediates bearing 130,000 molecules of iC3b or C3d, whereas treatment with the monoclonal antibody alone had no effect. The capacity of peripheral blood B lymphocytes to bind EC3d was similarly inhibited by the combination of HB-5 and GaM. The possibility that HB-5 may interact with a site on CR2 that is distinct from the ligand binding site permitted the direct analysis of the capacity of the HB-5 antigen to bind to the C3 fragments. Protein A-containing Staphylococcus aureus particles to which HB-5 had been bound were incubated with detergent lysates of Raji cells and B lymphocytes under conditions that had been shown to be associated only with the binding of the 145,000 Mr antigen. These particles bearing HB-5 and antigen derived from either cell type were shown to adhere specifically to EiC3b and EC3d, demonstrating that transfer of the HB-5 antigen from CR2-bearing cells to S. aureus particles led to the acquisition of CR2 function by the particles. The additional findings that the relatively weak capacity of Raji cells to form rosettes with EC3b was inhibited by HB-5 and that the S. aureus particles bearing immunoadsorbed HB-5 antigen bound to EC3b indicated that the C3b-binding function of the CR1-negative Raji cell resides in CR2, rather than in other membrane proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barel M., Charriaut C., Frade R. Isolation and characterization of a C3b receptor-like molecule from membranes of a human B lymphoblastoid cell line (Raji). FEBS Lett. 1981 Dec 21;136(1):111–114. doi: 10.1016/0014-5793(81)81225-2. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo J. R., Ruddy S., Studer E. J., Conrad D. H. Complement receptor binding of C3b-coated cells treated with C3b inactivator, beta 1H globulin and trypsin. J Immunol. 1979 Aug;123(2):523–528. [PubMed] [Google Scholar]
- Cooper N. R. Immune adherence by the fourth component of complement. Science. 1969 Jul 25;165(3891):396–398. doi: 10.1126/science.165.3891.396. [DOI] [PubMed] [Google Scholar]
- Dobson N. J., Lambris J. D., Ross G. D. Characteristics of isolated erythrocyte complement receptor type one (CR1, C4b-C3b receptor) and CR1-specific antibodies. J Immunol. 1981 Feb;126(2):693–698. [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Structural heterogeneity of the C3b/C4b receptor (Cr 1) on human peripheral blood cells. J Exp Med. 1983 Jun 1;157(6):2160–2165. doi: 10.1084/jem.157.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A., Miller G. W., Nussenzweig V. Human lymphocytes bear membrane receptors for C3b and C3d. J Clin Invest. 1973 Dec;52(12):3239–3242. doi: 10.1172/JCI107525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Magrath I. T., Berger M., Hammer C. H., Novikovs L., Santaella M., Frank M. M. Complement receptor expression by neoplastic and normal human cells. J Immunol. 1983 Aug;131(2):899–905. [PubMed] [Google Scholar]
- Gelfand M. C., Frank M. M., Green I. A receptor for the third component of complement in the human renal glomerulus. J Exp Med. 1975 Oct 1;142(4):1029–1034. doi: 10.1084/jem.142.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Iida K., Nadler L., Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983 Oct 1;158(4):1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Pangburn M. K., Oldroyd R. G. Breakdown of C3 after complement activation. Identification of a new fragment C3g, using monoclonal antibodies. J Exp Med. 1982 Jul 1;156(1):205–216. doi: 10.1084/jem.156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambris J. D., Dobson N. J., Ross G. D. Isolation of lymphocyte membrane complement receptor type two (the C3d receptor) and preparation of receptor-specific antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1828–1832. doi: 10.1073/pnas.78.3.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Fearon D. T., Levine R. P. Action of the C3b-inactivator on the cell-bound C3b. J Immunol. 1979 Mar;122(3):759–765. [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Schreiber R. D., Müller-Eberhard H. J. Interaction of target cell-bound C3bi and C3d with human lymphocyte receptors. Enhancement of antibody-mediated cellular cytotoxicity. J Exp Med. 1981 Jun 1;153(6):1592–1603. doi: 10.1084/jem.153.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J Exp Med. 1982 Jan 1;155(1):96–110. doi: 10.1084/jem.155.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Polley M. J., Rabellino E. M., Grey H. M. Two different complement receptors on human lymphocytes. One specific for C3b and one specific for C3b inactivator-cleaved C3b. J Exp Med. 1973 Oct 1;138(4):798–811. doi: 10.1084/jem.138.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner O., Hammann K. P., Schmid H. U., Schulz T., Alsenz J., Dierich M. P. A comparative evaluation of receptor reactivities for C3b, iC3b, and C3d on Raji lymphoblastoid cells. Int Arch Allergy Appl Immunol. 1982;69(3):231–237. doi: 10.1159/000233176. [DOI] [PubMed] [Google Scholar]
- Schmitt M., Mussel H. H., Dierich M. P. Qualitative and quantitative assessment of C3-receptor reactivities on lymphoid and phagocytic cells. J Immunol. 1981 May;126(5):2042–2047. [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Fearon D. T., Gartland G. L., Cooper M. D. Expression of C3b receptors on human be cells and myelomonocytic cells but not natural killer cells. J Immunol. 1983 Apr;130(4):1668–1673. [PubMed] [Google Scholar]
- Wilson J. G., Tedder T. F., Fearon D. T. Characterization of human T lymphocytes that express the C3b receptor. J Immunol. 1983 Aug;131(2):684–689. [PubMed] [Google Scholar]
- Wong W. W., Wilson J. G., Fearon D. T. Genetic regulation of a structural polymorphism of human C3b receptor. J Clin Invest. 1983 Aug;72(2):685–693. doi: 10.1172/JCI111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]