Abstract
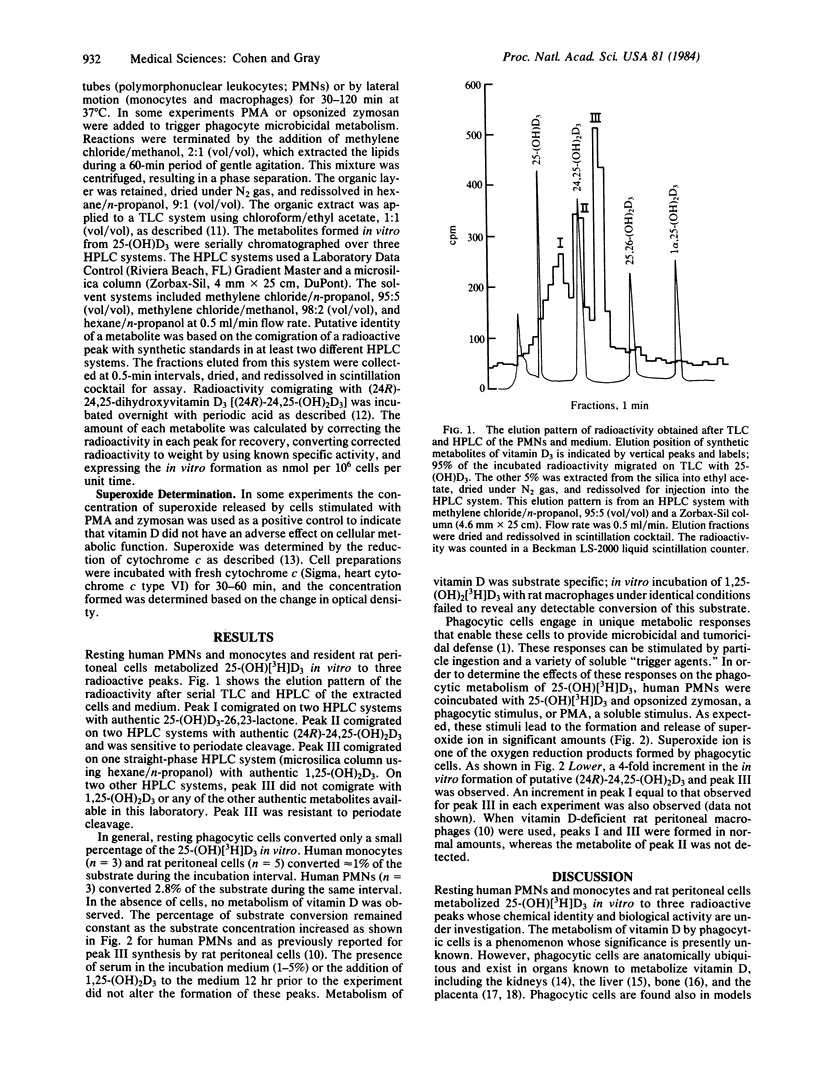
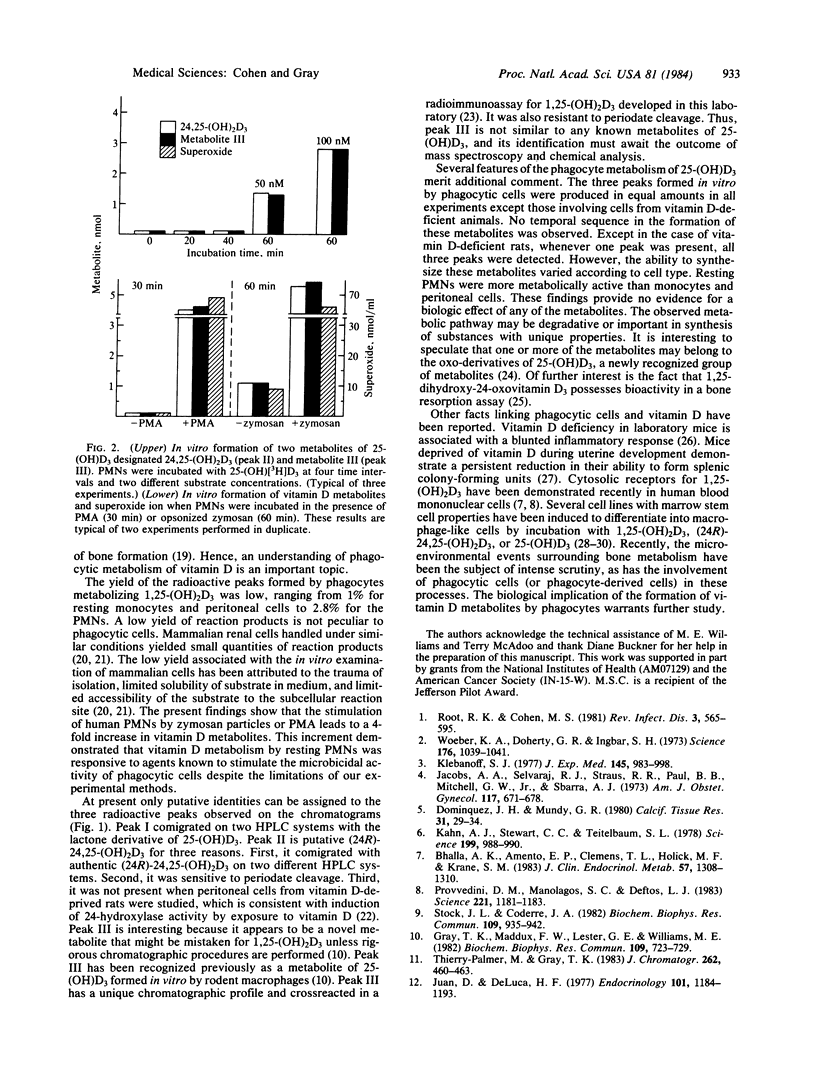
Phagocytic cells are widely distributed in tissues known to be important in the metabolism of vitamin D. Incubation of human polymorphonuclear leukocytes and monocytes and resident rat peritoneal macrophages with 3H-labeled 25-hydroxyvitamin D3 leads to the formation of three radioactive peaks. Peak I is most consistent with a lactone derivative of 25-hydroxyvitamin D3, and peak II has been identified as putative 24,25-dihydroxyvitamin D3. Peak III is a novel metabolite of 25-hydroxyvitamin D3 unlike any of the synthetic standards available in our laboratories. Human neutrophils converted more substrate than did the other phagocytes examined. The stimulation of neutrophils by opsonized zymosan or phorbol myristate acetate led to a 4-fold increase in synthesis of the metabolites. These results suggest that vitamin D metabolism by phagocytic cells may play a role in the microenvironmental events that surround bony metabolism and calcium homeostasis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Noff D., Edelstein S., Meyer M., Shibolet S., Goldman R. 1,25-dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int. 1981;33(6):673–676. doi: 10.1007/BF02409507. [DOI] [PubMed] [Google Scholar]
- Bhalla A. K., Amento E. P., Clemens T. L., Holick M. F., Krane S. M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983 Dec;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd R. C., Cohen M. S., Newman S. L., Gray T. K. Vitamin D metabolites change the phenotype of monoblastic U937 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7538–7541. doi: 10.1073/pnas.80.24.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez J. H., Mundy G. R. Monocytes mediate osteoclastic bone resorption by prostaglandin production. Calcif Tissue Int. 1980;31(1):29–34. doi: 10.1007/BF02407164. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gray T. K., Lester G. E., Lorenc R. S. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. 1979 Jun 22;204(4399):1311–1313. doi: 10.1126/science.451538. [DOI] [PubMed] [Google Scholar]
- Gray T. K., Maddux F. W., Lester G. E., Williams M. E. Rodent macrophages metabolize 25-hydroxyvitamin D3 in vitro. Biochem Biophys Res Commun. 1982 Dec 15;109(3):723–729. doi: 10.1016/0006-291x(82)92000-9. [DOI] [PubMed] [Google Scholar]
- Gray T. K., McAdoo T. Radioimmunoassay for 1,25-dihydroxycholecalciferol. Clin Chem. 1983 Jan;29(1):196–200. [PubMed] [Google Scholar]
- Jacobs A. A., Selvardj R. J., Strauss R. R., Paul B. B., Mitchell G. W., Jr, Sbarra A. J. The role of the phagocyte in host-parasite interactions. XXXIX. Stimulation of bactericidal activity of myeloperoxidase-containing leukocytic fractions by estrogens. Am J Obstet Gynecol. 1973 Nov 1;117(5):671–678. doi: 10.1016/0002-9378(73)90209-3. [DOI] [PubMed] [Google Scholar]
- Juan D., DeLUCA H. F. The regulation of 24,25-dihydroxyvitamin D3 production in cultures of monkey kidney cells. Endocrinology. 1977 Oct;101(4):1184–1193. doi: 10.1210/endo-101-4-1184. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Stewart C. C., Teitelbaum S. L. Contact-mediated bone resorption by human monocytes in vitro. Science. 1978 Mar 3;199(4332):988–990. doi: 10.1126/science.622581. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Estrogen binding by leukocytes during phagocytosis,. J Exp Med. 1977 Apr 1;145(4):983–998. doi: 10.1084/jem.145.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E., Reddy G. S., Chandraratna R. A., Okamura W. H., Kruse J. R., Popjàk G., Bishop J. E., Norman A. W. 23,25-Dihydroxy-24-oxovitamin D3: a metabolite of vitamin D3 made in the kidney. Biochemistry. 1983 Apr 12;22(8):1798–1805. doi: 10.1021/bi00277a009. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Nomura H., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 suppresses proliferation of murine granulocyte-macrophage progenitor cells (CFU-C). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1728–1733. doi: 10.1016/s0006-291x(82)80111-3. [DOI] [PubMed] [Google Scholar]
- Olson E. B., Jr, Knutson J. C., Bhattacharyya M. H., DeLuca H. F. The effect of hepatectomy on the synthesis of 25-hydroxyvitamin D3. J Clin Invest. 1976 May;57(5):1213–1220. doi: 10.1172/JCI108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J., Manolagas S. C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Reddi A. H. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981 Feb;1(2):209–226. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Stock J. L., Coderre J. A. Calcitonin and parathyroid hormone inhibit accumulation of cyclic AMP in stimulated human mononuclear cells. Biochem Biophys Res Commun. 1982 Dec 15;109(3):935–942. doi: 10.1016/0006-291x(82)92030-7. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Halloran B., Schnoes H. K., DeLuca H. F. In vitro production of 1,25-dihydroxyvitamin D3 by rat placental tissue. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5033–5035. doi: 10.1073/pnas.76.10.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry-Palmer M., Gray T. K. Separation of hydroxylated metabolites of vitamin D3 by high-performance thin-layer chromatography. J Chromatogr. 1983 Jun 24;262:460–463. doi: 10.1016/s0021-9673(01)88141-3. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Bottemiller B. L., Howard G. A., Baylink D. J. In vitro metabolism of 25-hydroxyvitamin D3 by isolated rat kidney cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1537–1540. doi: 10.1073/pnas.77.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. T., Puzas J. E., Forte M. D., Lester G. E., Gray T. K., Howard G. A., Baylink D. J. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A., Doherty G. F., Ingbar S. H. Stimulation by phagocytosis of the deiodination of L-thyroxine in human leukocytes. Science. 1972 Jun 2;176(4038):1039–1041. doi: 10.1126/science.176.4038.1039. [DOI] [PubMed] [Google Scholar]


