Abstract
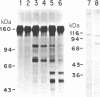
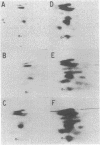
Purified preparations of epidermal growth factor (EGF) receptor were used to test hen oviduct progesterone receptor subunits as substrates for phosphorylation catalyzed by EGF receptor. Both the 80-kilodalton (kDa) (A) and the 105-kDa (B) progesterone receptor subunits were phosphorylated in a reaction that required EGF and EGF receptor. No phosphorylation of progesterone receptor subunits was observed in the absence of EGF receptor, even when Ca2+ was substituted for Mg2+ and Mn2+. Phospho amino acid analysis revealed phosphorylation at tyrosine residues, with no phosphorylation detectable at serine or threonine residues. Two-dimensional maps of phosphopeptides generated from phosphorylated 80- or 105-kDa subunits by tryptic digestion revealed similar patterns, with resolution of two major, several minor, and a number of very minor phosphopeptides. The Km of progesterone receptor for phosphorylation by EGF-activated EGF receptor was 100 nM and the Vmax was 2.5 nmol/min per mg of EGF receptor protein at 0 degrees C. The stoichiometry of phosphorylation/hormone binding for progesterone receptor subunits was 0.31 at ice-bath temperature and approximately 1.0 at 22 degrees C.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Barsh G. S., Carney D. H., Cunningham D. D. Dexamethasone modulates binding and action of epidermal growth factor in serum-free cell culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1882–1886. doi: 10.1073/pnas.75.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Grego B., Hearn M. T., Knesel J. A., Morgan F. J., Simpson R. J. Phosphorylation of human growth hormone by the epidermal growth factor-stimulated tyrosine kinase. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5276–5280. doi: 10.1073/pnas.80.17.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Knesel J., Monckton J. M. Phosphorylation of gastrin-17 by epidermal growth factor-stimulated tyrosine kinase. Nature. 1983 Feb 3;301(5899):435–437. doi: 10.1038/301435a0. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Stanley I. J., Nice E. C. A synthetic peptide containing the autophosphorylation site of the transforming protein of Harvey sarcoma virus is phosphorylated by the EGF-stimulated tyrosine kinase. FEBS Lett. 1983 Mar 21;153(2):257–261. doi: 10.1016/0014-5793(83)80619-x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M., Schrader W. T., O'Malley B. W. Assessment of structural similarities in chick oviduct progesterone receptor subunits by partial proteolysis of photoaffinity-labeled proteins. J Biol Chem. 1983 Jun 25;258(12):7331–7337. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Rowley D. R., Lobl T. J., Tindall D. J. Purification and characterization of androgen receptor from steer seminal vesicle. Biochemistry. 1982 Aug 17;21(17):4102–4109. doi: 10.1021/bi00260a029. [DOI] [PubMed] [Google Scholar]
- Chinkers M., Cohen S. Purified EGF receptor-kinase interacts specifically with antibodies to Rous sarcoma virus transforming protein. Nature. 1981 Apr 9;290(5806):516–519. doi: 10.1038/290516a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Fava R. A., Sawyer S. T. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6237–6241. doi: 10.1073/pnas.79.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Discrete primary locations of a tyrosine-protein kinase and of three proteins that contain phosphotyrosine in virally transformed chick fibroblasts. J Cell Biol. 1982 Aug;94(2):287–296. doi: 10.1083/jcb.94.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coty W. A. Reversible dissociation of steroid hormone x receptor complexes by mercurial reagents. J Biol Chem. 1980 Sep 10;255(17):8035–8037. [PubMed] [Google Scholar]
- Dure L. S., 4th, Schrader W. T., O'Malley B. W. Covalent attachment of a progestational steroid to chick oviduct progesterone receptor by photoaffinity labelling. Nature. 1980 Feb 21;283(5749):784–786. doi: 10.1038/283784a0. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Erikson E., Shealy D. J., Erikson R. L. Evidence that viral transforming gene products and epidermal growth factor stimulate phosphorylation of the same cellular protein with similar specificity. J Biol Chem. 1981 Nov 25;256(22):11381–11384. [PubMed] [Google Scholar]
- Erneux C., Cohen S., Garbers D. L. The kinetics of tyrosine phosphorylation by the purified epidermal growth factor receptor kinase of A-431 cells. J Biol Chem. 1983 Apr 10;258(7):4137–4142. [PubMed] [Google Scholar]
- Garcia T., Tuohimaa P., Mester J., Buchou T., Renoir J. M., Baulieu E. E. Protein kinase activity of purified components of the chicken oviduct progesterone receptor. Biochem Biophys Res Commun. 1983 Jun 29;113(3):960–966. doi: 10.1016/0006-291x(83)91092-6. [DOI] [PubMed] [Google Scholar]
- Ghosh-Dastidar P., Fox C. F. Epidermal growth factor and epidermal growth factor receptor-dependent phosphorylation of a Mr = 34,000 protein substrate for pp60src. J Biol Chem. 1983 Feb 10;258(3):2041–2044. [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Costlow M. E., McGuire W. L. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975 Dec;26(6):785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Pratt W. B. Direct demonstration of glucocorticoid receptor phosphorylation by intact L-cells. J Biol Chem. 1983 Apr 10;258(7):4630–4635. [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Kudlow J. E., Buss J. E., Gill G. N. Anti-pp60src antibodies are substrates for EGF-stimulated protein kinase. Nature. 1981 Apr 9;290(5806):519–521. doi: 10.1038/290519a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Dahmer M. K., Hammond N. D., Sando J. J., Pratt W. B. Molybdate inhibition of glucocorticoid receptor inactivation and transformation. J Biol Chem. 1979 Dec 10;254(23):11884–11890. [PubMed] [Google Scholar]
- Linsley P. S., Fox C. F. Direct linkage of EGF to its receptor: characterization and biological relevance. J Supramol Struct. 1980;14(4):441–459. doi: 10.1002/jss.400140404. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborene C. K., Hamilton B., Nover M. Receptor binding and processing of epidermal growth factor by human breast cancer cells. J Clin Endocrinol Metab. 1982 Jul;55(1):86–93. doi: 10.1210/jcem-55-1-86. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Pike L. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Epidermal growth factor stimulates the phosphorylation of synthetic tyrosine-containing peptides by A431 cell membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1443–1447. doi: 10.1073/pnas.79.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri R. K., Grandics P., Dougherty J. J., Toft D. O. Purification of "nontransformed" avian progesterone receptor and preliminary characterization. J Biol Chem. 1982 Sep 25;257(18):10831–10837. [PubMed] [Google Scholar]
- Renoir J. M., Yang C. R., Formstecher P., Lustenberger P., Wolfson A., Redeuilh G., Mester J., Richard-Foy H., Baulieu E. E. Progesterone receptor from chick oviduct: purification of molybdate-stabilized form and preliminary characterization. Eur J Biochem. 1982 Sep;127(1):71–79. doi: 10.1111/j.1432-1033.1982.tb06839.x. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Sando J. J., La Forest A. C., Pratt W. B. ATP-dependent activation of L cell glucocorticoid receptors to the steroid binding form. J Biol Chem. 1979 Jun 10;254(11):4772–4778. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Enhancement of polyoma virus middle T antigen tyrosine phosphorylation by epidermal growth factor. Nature. 1983 Aug 25;304(5928):742–744. doi: 10.1038/304742a0. [DOI] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Weigel N. L., Tash J. S., Means A. R., Schrader W. T., O'Malley B. W. Phosphorylation of hen progesterone receptor by cAMP dependent protein kinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):513–519. doi: 10.1016/0006-291x(81)91549-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. In vitro phosphorylation of angiotensin analogs by tyrosyl protein kinases. J Biol Chem. 1983 Jan 25;258(2):1022–1025. [PubMed] [Google Scholar]