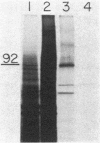
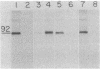
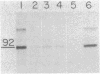
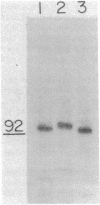
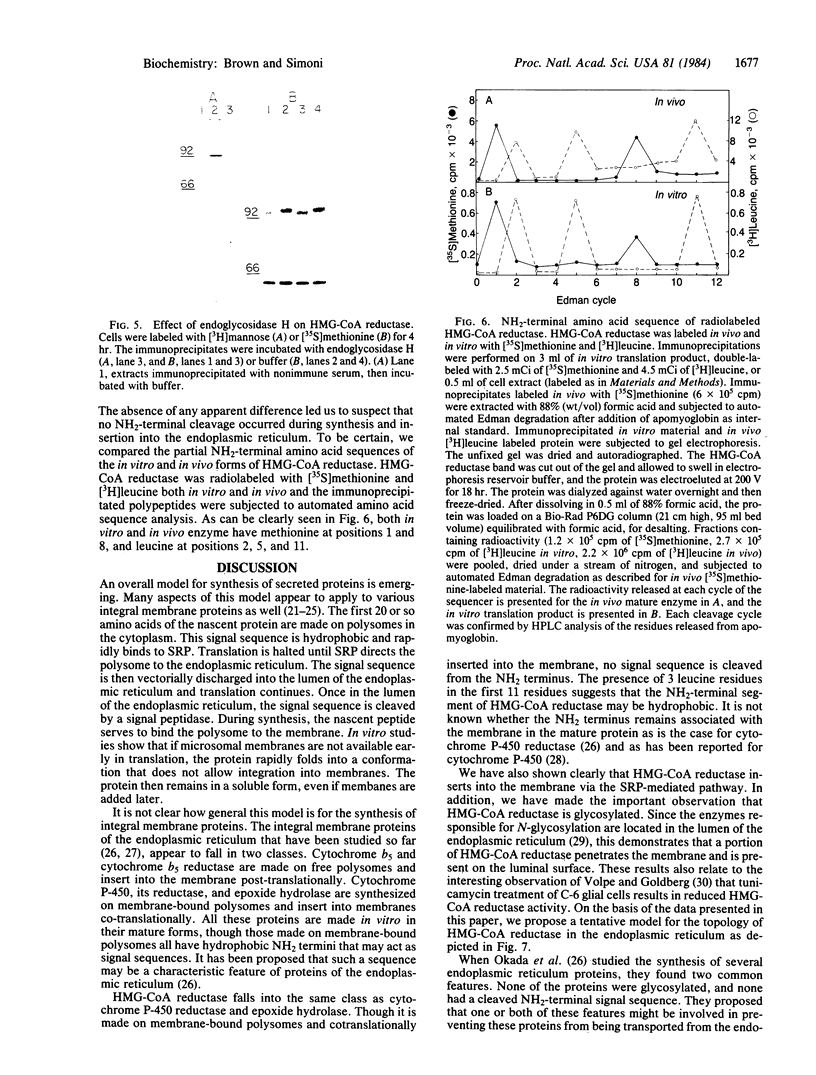
Abstract
Using a cell line, C100, that overproduces 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase; EC 1.1.1.34) 100-fold, we have studied the synthesis and insertion of this protein into the endoplasmic reticulum. The enzyme is synthesized on membrane-bound polysomes. It is cotranslationally but not post-translationally inserted into dog pancreatic microsomes. This cotranslational insertion is dependent upon signal recognition particle. HMG-CoA reductase is glycosylated with an oligosaccharide(s) of the "high-mannose" type sensitive to endo-beta-D-N-acetylglucosaminidase H. Partial determination of the NH2-terminal amino acid sequence of the in vitro translation product and the mature polypeptide indicate they are the same and demonstrate there is no cleavage of an NH2-terminal signal sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nun S., Kreibich G., Adesnik M., Alterman L., Negishi M., Sabatini D. D. Synthesis and insertion of cytochrome P-450 into endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):965–969. doi: 10.1073/pnas.77.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S. D., French J. S., Williams C. H., Jr, Coon M. J. Role of a hydrophobic polypeptide in the N-terminal region of NADPH-cytochrome P-450 reductase in complex formation with P-450LM. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1528–1535. doi: 10.1016/0006-291x(79)91238-5. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Dietschy J. M., Siperstein M. D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase. Solubilization and purification of a cold-sensitive microsomal enzyme. J Biol Chem. 1973 Jul 10;248(13):4731–4738. [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Faust J. R., MacDonald R. J., Brown M. S., Goldstein J. L. Molecular cloning of 3-hydroxy-3-methylglutaryl coenzyme a reductase and evidence for regulation of its mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7704–7708. doi: 10.1073/pnas.79.24.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A., Lan S. F., Tanaka R. D., Fogelman A. M. Mevalonolactone inhibits the rate of synthesis and enhances the rate of degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in rat hepatocytes. J Biol Chem. 1983 Jun 25;258(12):7272–7275. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. N-Linked glycoprotein assembly. Evidence that oligosaccharide attachment occurs within the lumen of the endoplasmic reticulum. J Biol Chem. 1980 Apr 25;255(8):3600–3604. [PubMed] [Google Scholar]
- Hardeman E. C., Jenke H. S., Simoni R. D. Overproduction of a Mr 92,000 protomer of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in compactin-resistant C100 cells. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1516–1520. doi: 10.1073/pnas.80.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Liscum L., Cummings R. D., Anderson R. G., DeMartino G. N., Goldstein J. L., Brown M. S. 3-Hydroxy-3-methylglutaryl-CoA reductase: a transmembrane glycoprotein of the endoplasmic reticulum with N-linked "high-mannose" oligosaccharides. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7165–7169. doi: 10.1073/pnas.80.23.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskey K. L., Faust J. R., Chin D. J., Brown M. S., Goldstein J. L. Amplification of the gene for 3-hydroxy-3-methylglutaryl coenzyme A reductase, but not for the 53-kDa protein, in UT-1 cells. J Biol Chem. 1983 Jul 10;258(13):8462–8469. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Ness G. C., Way S. C., Wickham P. S. Proteinase involvement in the solubilization of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):81–85. doi: 10.1016/0006-291x(81)91491-1. [DOI] [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H., Arbogast L. Y., Ouellette L., Howard B. V. Preparation of delipidized serum protein for use in cell culture systems. In Vitro. 1976 Aug;12(8):554–557. doi: 10.1007/BF02797438. [DOI] [PubMed] [Google Scholar]
- Ryan J., Hardeman E. C., Endo A., Simoni R. D. Isolation and characterization of cells resistant to ML236B (compactin) with increased levels of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 1981 Jul 10;256(13):6762–6768. [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Goldberg R. I. Effect of tunicamycin on 3-hydroxy-3-methylglutaryl coenzyme A reductase in C-6 glial cells. J Biol Chem. 1983 Aug 10;258(15):9220–9226. [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Dobberstein B. Protein transfer across microsomal membranes reassembled from separated membrane components. Nature. 1978 Jun 15;273(5663):569–571. doi: 10.1038/273569a0. [DOI] [PubMed] [Google Scholar]