Abstract
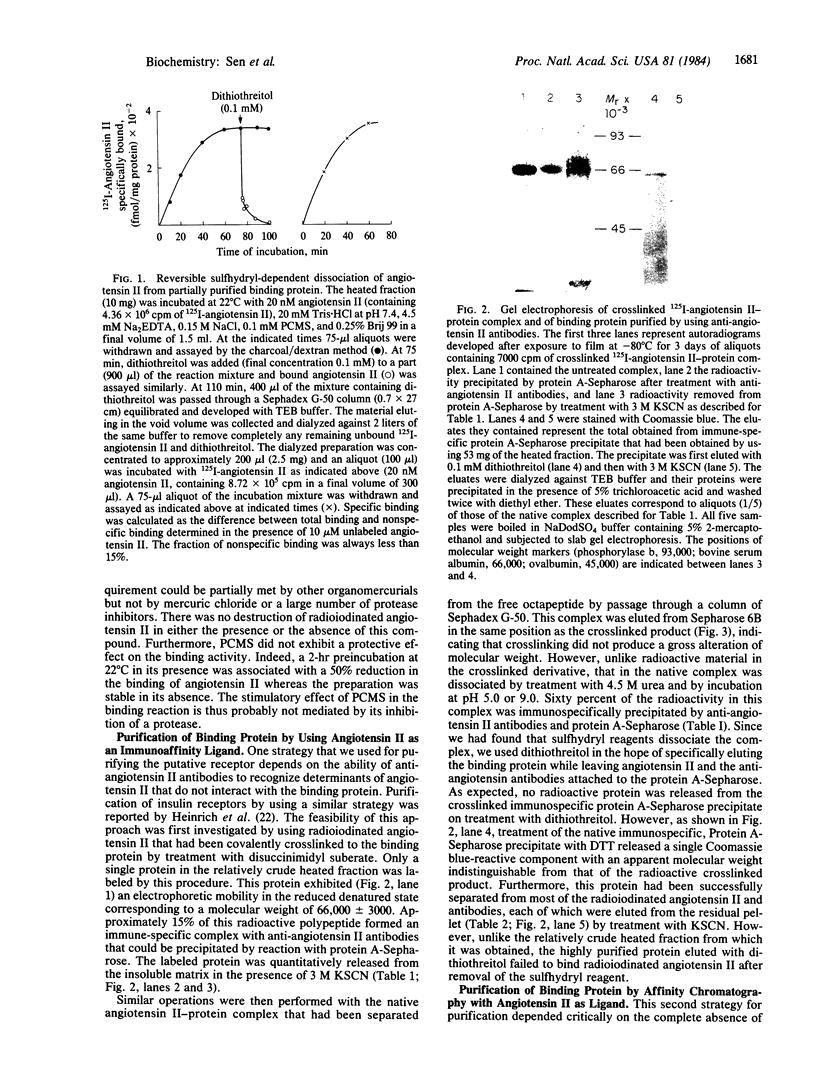
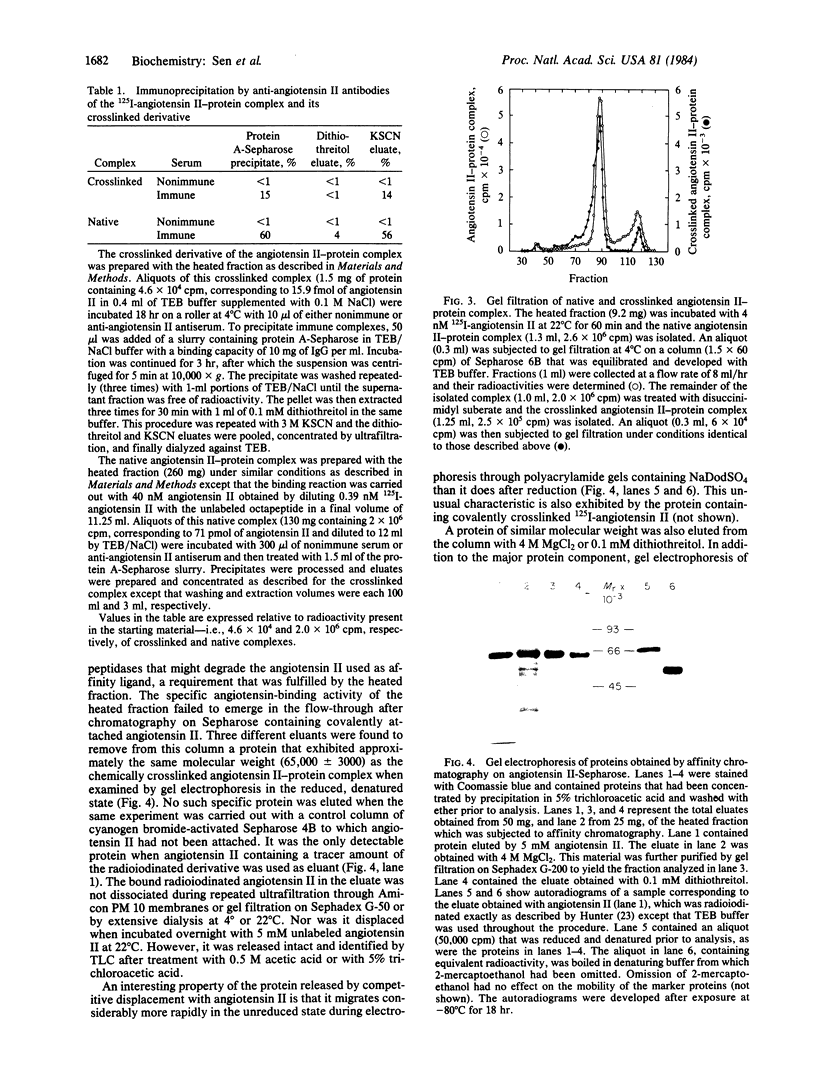
A protein that specifically binds angiotensin II has been isolated in nearly homogeneous form by two independent approaches after solubilization from rabbit liver particles by treatment with digitonin. The protein purified by either of these methods resembles in size the single radioactive macromolecular component made by using disuccinimidyl suberate to crosslink radioiodinated angiotensin II with its receptor in the solubilized extract. In the first technique, angiotensin II as an affinity ligand specifically extracted the protein from a preparation that had been freed of angiotensin-degrading activity. In the second approach, the angiotensin II-protein complex was specifically precipitated by anti-angiotensin II antibodies and staphylococcal protein A-Sepharose. The protein could be eluted from the affinity column with angiotensin II or 4 M MgCl2. The angiotensin II-protein complex dissociated in the presence of sulfhydryl-containing reagents, and these could therefore be used to elute it from either the chemical or the immunoaffinity-based matrix. This effect of sulfhydryl-containing reagents and the paradoxical observation that the isolated protein after denaturation exhibited a slower electrophoretic mobility in its reduced form that in its unreduced form suggest that the binding configuration of this protein may be sensitive to reduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CARPENTER C. C., DAVIS J. O., AYERS C. R. Relation of renin, angiotensin II, and experimental renal hypertension to aldosterone secretion. J Clin Invest. 1961 Nov;40:2026–2042. doi: 10.1172/JCI104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanile C. P., Crane J. K., Peach M. J., Garrison J. C. The hepatic angiotensin II receptor. I. Characterization of the membrane-binding site and correlation with physiological response in hepatocytes. J Biol Chem. 1982 May 10;257(9):4951–4958. [PubMed] [Google Scholar]
- Capponi A. M., Catt K. J. Angiotensin II receptors in adrenal cortex and uterus. Binding and activation properties of angiotensin analogues. J Biol Chem. 1979 Jun 25;254(12):5120–5127. [PubMed] [Google Scholar]
- Capponi A. M., Catt K. J. Solubilization and characterization of adrenal and uterine angiotensin II receptors after photoaffinity labeling. J Biol Chem. 1980 Dec 25;255(24):12081–12086. [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J., Keegan M. E. Inactivation of angiotensin II receptors in bovine adrenal cortex by dithiothreitol: further evidence for the essential nature of disulfide bonds. Biochem Pharmacol. 1982 May 15;31(10):1903–1906. doi: 10.1016/0006-2952(82)90495-6. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Solubilization of angiotensin II receptors in bovine adrenal cortex. Life Sci. 1981 Aug 10;29(6):613–618. doi: 10.1016/0024-3205(81)90439-2. [DOI] [PubMed] [Google Scholar]
- Douglas J., Saltman S., Fredlund P., Kondo T., Catt K. J. Receptor binding of angiotensin II and antagonists. Correlation with aldosterone production by isolated canine adrenal glomerulosa cells. Circ Res. 1976 Jun;38(6 Suppl 2):108–112. doi: 10.1161/01.res.38.6.108. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Stafford S. S., Schaefer M. L., Ho H., La Vorgna K. A., Jamieson J. D. Biologically active derivatives of angiotensin for labeling cellular receptors. J Med Chem. 1978 Dec;21(12):1279–1283. doi: 10.1021/jm00210a020. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K., Florio V. A., Twible D. A. The role of calcium ion as a mediator of the effects of angiotensin II, catecholamines, and vasopressin on the phosphorylation and activity of enzymes in isolated hepatocytes. J Biol Chem. 1979 Aug 10;254(15):7147–7156. [PubMed] [Google Scholar]
- Gocke D. J., Gerten J., Sherwood L. M., Laragh J. H. Physiological and pathological variations of plasma angiotensin II in man. Correlation with renin activity and sodium balance. Circ Res. 1969 May;24(5 Suppl):131–148. [PubMed] [Google Scholar]
- HELMER O. M. Differentiation between two forms of angiotonin by means of spirally cut strips of rabbit aorta. Am J Physiol. 1957 Mar;188(3):571–577. doi: 10.1152/ajplegacy.1957.188.3.571. [DOI] [PubMed] [Google Scholar]
- Heinrich J., Pilch P. F., Czech M. P. Purification of the adipocyte insulin receptor by immunoaffinity chromatography. J Biol Chem. 1980 Feb 25;255(4):1732–1737. [PubMed] [Google Scholar]
- LARAGH J. H., ANGERS M., KELLY W. G., LIEBERMAN S. Hypotensive agents and pressor substances. The effect of epinephrine, norepinephrine, angiotensin II, and others on the secretory rate of aldosterone in man. JAMA. 1960 Sep 17;174:234–240. doi: 10.1001/jama.1960.03030030014003. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Goodfriend T. L. Angiotensin receptors. Am J Physiol. 1970 May;218(5):1319–1328. doi: 10.1152/ajplegacy.1970.218.5.1319. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Cushman D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977 Apr 22;196(4288):441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- Paglin S., Jamieson J. D. Covalent crosslinking of angiotensin II to its binding sites in rat adrenal membranes. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3739–3743. doi: 10.1073/pnas.79.12.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett A. A., Harris E., Tristram E. W., Wyvratt M. J., Wu M. T., Taub D., Peterson E. R., Ikeler T. J., ten Broeke J., Payne L. G. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980 Nov 20;288(5788):280–283. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- Phillips M. I. Angiotensin in the brain. Neuroendocrinology. 1978;25(6):354–377. doi: 10.1159/000122756. [DOI] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, KAHN J. R., SHUMWAY N. P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956 Mar 1;103(3):295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen I., Jim K. F., Soffer R. L. Solubilization and characterization of an angiotensin II binding protein from liver. Eur J Biochem. 1983 Oct 17;136(1):41–49. doi: 10.1111/j.1432-1033.1983.tb07702.x. [DOI] [PubMed] [Google Scholar]