Abstract
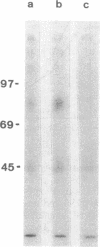
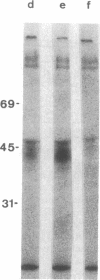
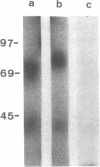
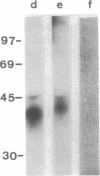
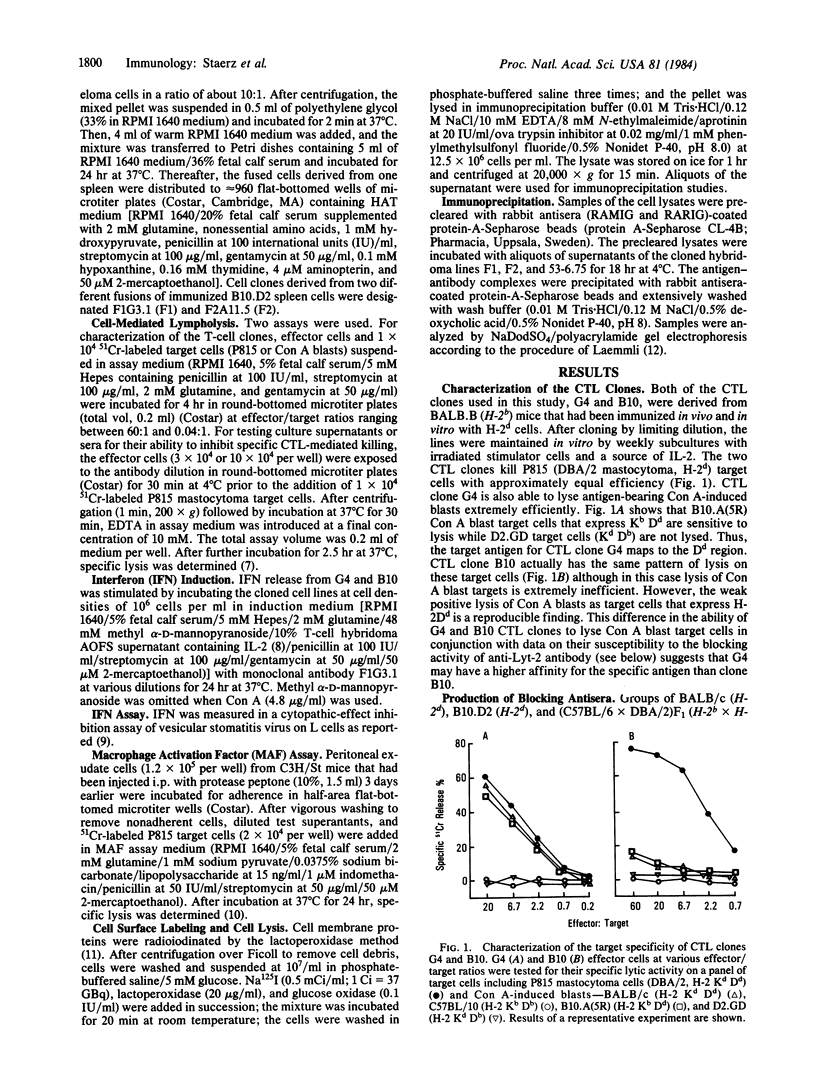
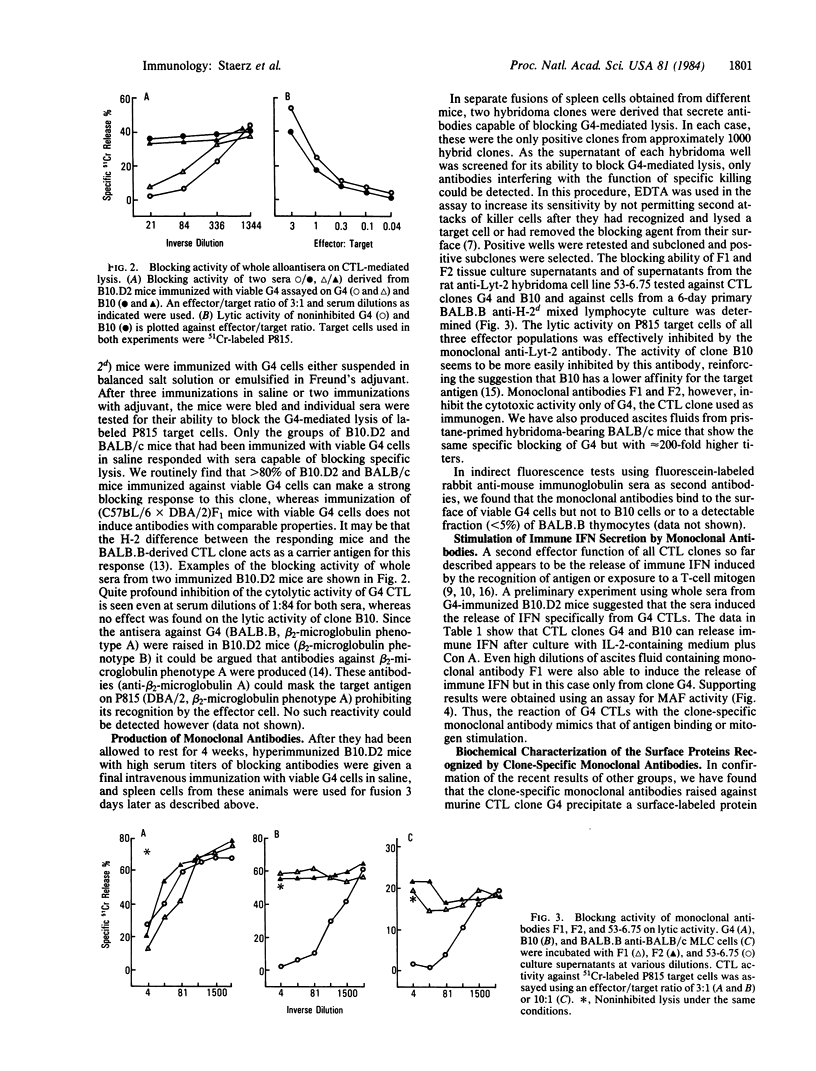
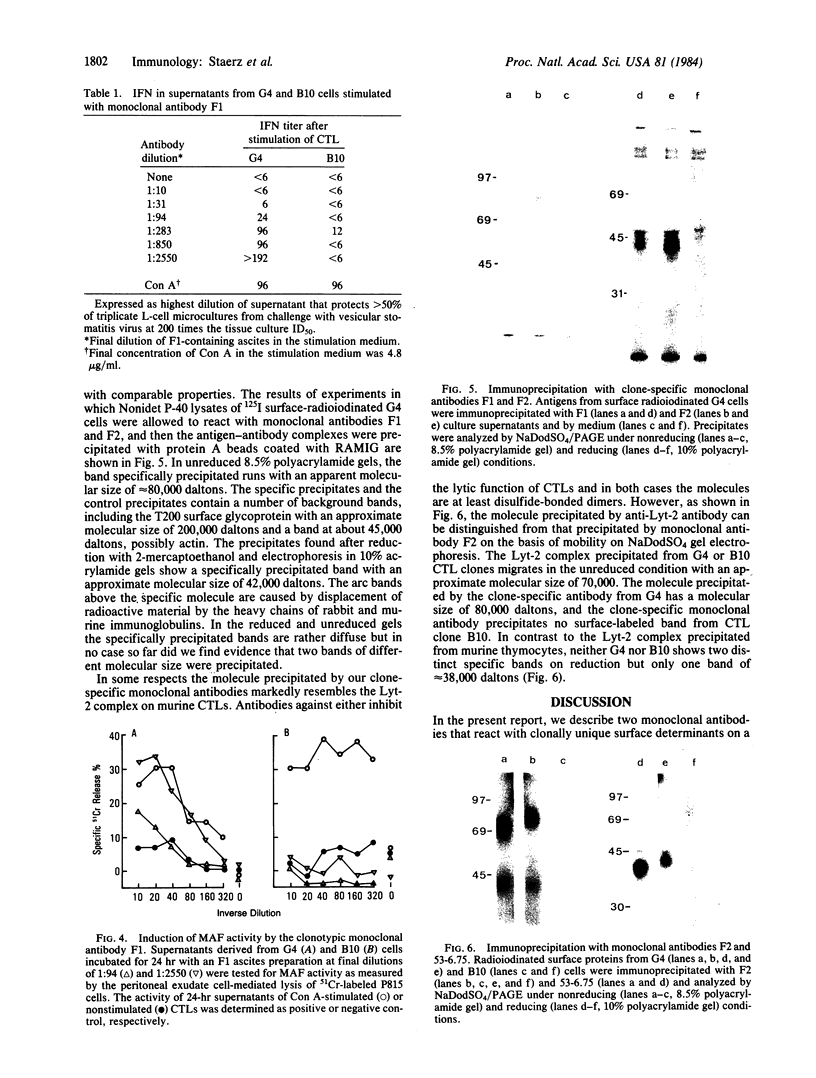
Two antibody-secreting murine hybridomas, F1G3.1 and F2A11.5, have been established from B10.D2 mice immunized with cells from the murine cytotoxic T-lymphocyte clone G4. The two clones used, G4 and B10, were derived from BALB.B (H-2b) mice and the target antigen specificity of both maps to the Dd region of the murine H-2 complex. However, B10 has a lower affinity for the target cells, as shown by its lower specific killing of blasts and its higher susceptibility to blocking by anti-Lyt-2 monoclonal antibody 53-6.75. The monoclonal antibodies, F1G3.1 and F2A11.5, react only with cells from clone G4. Similarly, they block only the specific cytolysis mediated by G4; no effect on cytotoxicity mediated by B10 or by heterogeneous populations of cytotoxic T lymphocytes was found. F1G3.1, especially, is very active in stimulating G4 to secrete immune interferon; B10 in contrast did not show any induction on treatment with these monoclonal antibodies. The structure of the surface antigen on G4 cells recognized by these monoclonal antibodies was revealed by immunoprecipitation studies of radioiodinated cell surface proteins. A protein dimer could be identified with an apparent molecular size of 80,000 daltons consisting of monomers migrating as 42,000-dalton proteins on reduction. So far, electrophoresis in the presence of NaDodSO4 does not indicate any heterogeneity in the size of the monomers. This molecule can be distinguished from the Lyt-2 complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., McIntyre B. W., Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982 Nov;129(5):2293–2300. [PubMed] [Google Scholar]
- Glasebrook A. L., Kelso A., MacDonald H. R. Cytolytic T lymphocyte clones that proliferate autonomously to specific alloantigenic stimulation. II. Relationship of the Lyt-2 molecular complex to cytolytic activity, proliferation, and lymphokine secretion. J Immunol. 1983 Apr;130(4):1545–1551. [PubMed] [Google Scholar]
- Harwell L., Skidmore B., Marrack P., Kappler J. Concanavalin A-inducible, interleukin-2-producing T cell hybridoma. J Exp Med. 1980 Oct 1;152(4):893–904. doi: 10.1084/jem.152.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., Kubo R., White J., Pigeon M., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983 Apr 1;157(4):1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Palladino M. A., Khoury G., Old L. J. Mouse Lyt-2 antigen: evidence for two heterodimers with a common subunit. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2654–2657. doi: 10.1073/pnas.79.8.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L., Kanagawa O., Brunner K. T. Production of macrophage-activating factor by T lymphocyte clones and correlation with other lymphokine activities. J Immunol. 1982 Aug;129(2):550–556. [PubMed] [Google Scholar]
- Klein J. R., Raulet D. H., Pasternack M. S., Bevan M. J. Cytotoxic T lymphocytes produce immune interferon in response to antigen or mitogen. J Exp Med. 1982 Apr 1;155(4):1198–1203. doi: 10.1084/jem.155.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancki D. W., Lorber M. I., Loken M. R., Fitch F. W. A clone-specific monoclonal antibody that inhibits cytolysis of a cytolytic T cell clone. J Exp Med. 1983 Mar 1;157(3):921–935. doi: 10.1084/jem.157.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Shimonkevitz R., Hannum C., Haskins K., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. IV. An antiidiotypic antibody predicts both antigen and I-specificity. J Exp Med. 1983 Nov 1;158(5):1635–1646. doi: 10.1084/jem.158.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson J. Genetics of beta-2 microglobulin in the mouse. Immunogenetics. 1983;17(3):219–260. doi: 10.1007/BF00364409. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Lin Y. L., Askonas B. A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982 Jan 14;295(5845):150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Acuto O., Schlossman S. F. Comparison of T3-associated 49- and 43-kilodalton cell surface molecules on individual human T-cell clones: evidence for peptide variability in T-cell receptor structures. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4104–4108. doi: 10.1073/pnas.80.13.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Haskins K. M., Marrack P., Kappler J. Use of I region-restricted, antigen-specific T cell hybridomas to produce idiotypically specific anti-receptor antibodies. J Immunol. 1983 Mar;130(3):1033–1037. [PubMed] [Google Scholar]