Abstract
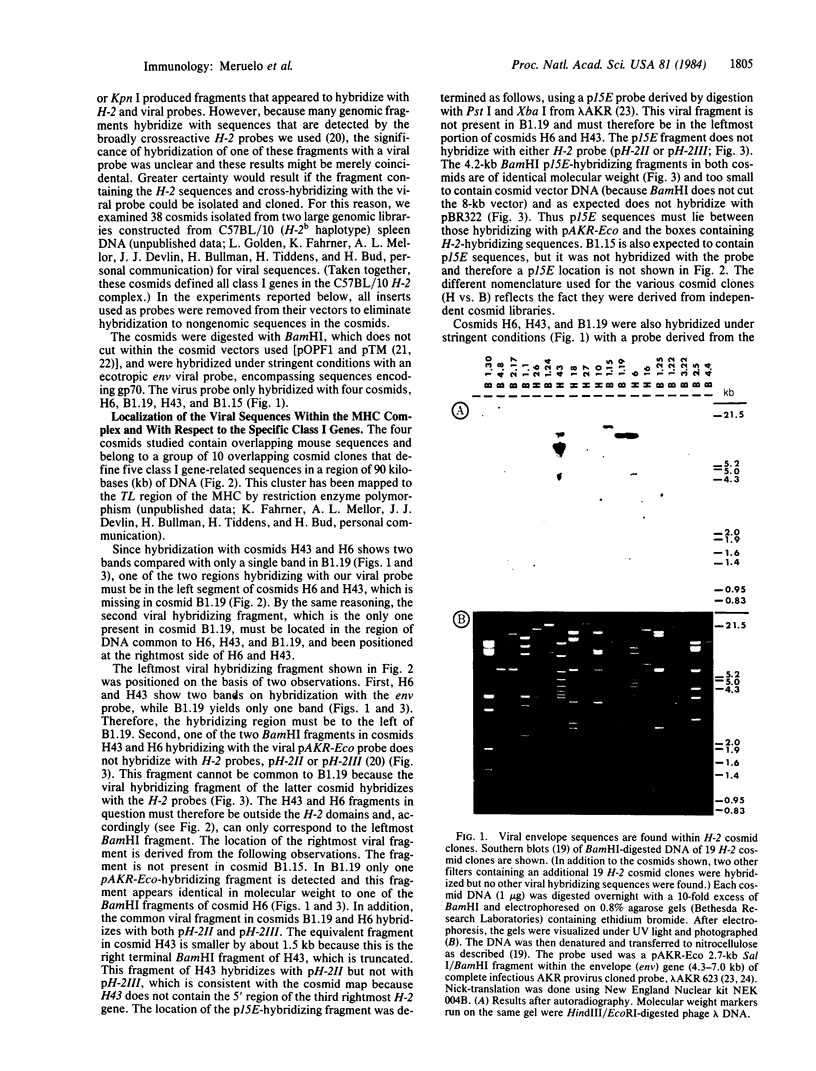
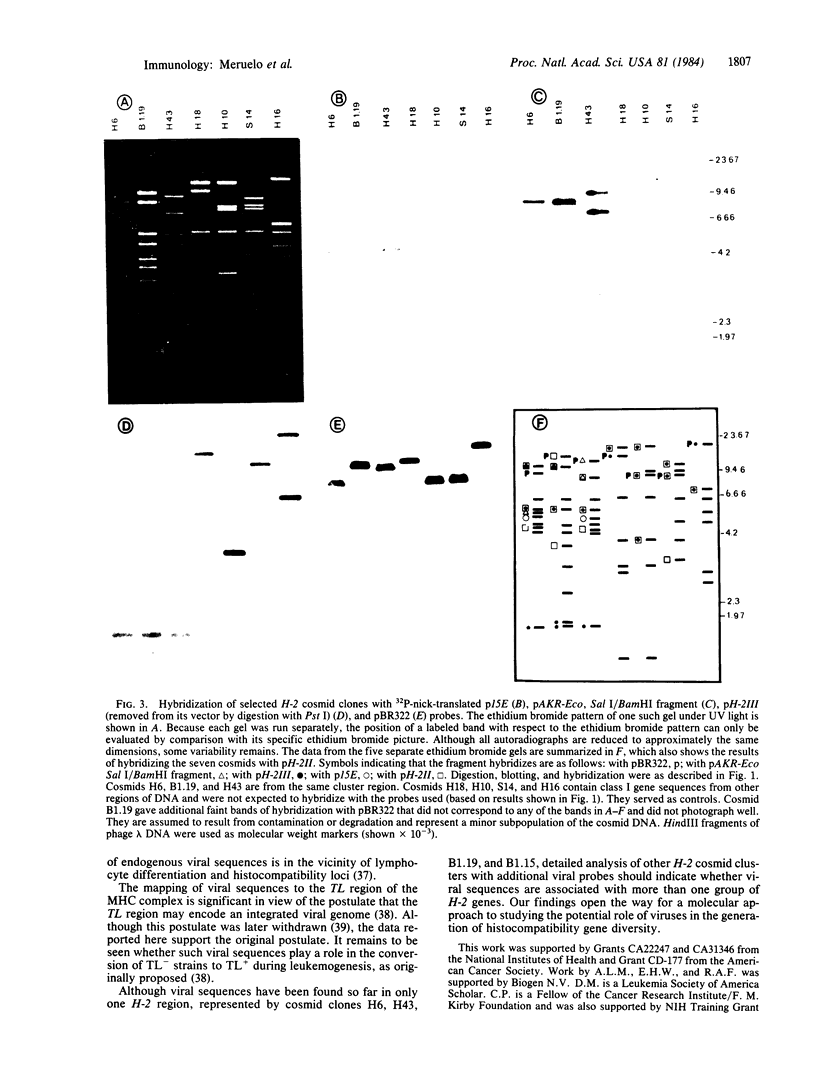
The studies reported here localize murine leukemia viral sequences to the TL region of the major histocompatibility complex, H-2. We examined a battery of 38 cosmids, isolated from two large genomic libraries constructed from C57BL/10 spleen DNA, that define 25 class I gene sequences. The viral probes used hybridized with only four cosmids, containing overlapping mouse sequences, that define four class I gene-related sequences in a region of 90 kilobases of DNA. The data show that two distinct viral envelope sequences are contained in the cluster. One of these sequences is situated with its 3' end next to the 3' end of a class I sequence. The other sequence, which does not contain the entire viral envelope, is proximal to the 3' end of a different class I sequence. Hybridization of the viral probes with the H-2 cosmid clones does not appear to be due to homology between viral and H-2 sequences. Rather, the viral sequences detected appear to be linked to or inserted amid class I genes. These findings may be significant in understanding molecular mechanisms involved in the generation of H-2 class I gene diversity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W. Heritable histocompatibility changes: lysogeny in mice? Transplantation. 1966 Jul;4(4):482–488. doi: 10.1097/00007890-196607000-00012. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Blatt C., Mileham K., Haas M., Nesbitt M. N., Harper M. E., Simon M. I. Chromosomal mapping of the mink cell focus-inducing and xenotropic env gene family in the mouse. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6298–6302. doi: 10.1073/pnas.80.20.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. F., Gelmann E. P., Reitz M. S., Jr Homology of human T-cell leukaemia virus envelope gene with class I HLA gene. Nature. 1983 Sep 1;305(5929):60–62. doi: 10.1038/305060a0. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Bodmer W. F. Protein clinical manifestations of primary tumors of the heart. Am J Med. 1972 Jan;52(1):1–8. doi: 10.1016/0002-9343(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Golden L., Weiss E., Bullman H., Hurst J., Simpson E., James R. F., Townsend A. R., Taylor P. M., Schmidt W. Expression of murine H-2Kb histocompatibility antigen in cells transformed with cloned H-2 genes. Nature. 1982 Aug 5;298(5874):529–534. doi: 10.1038/298529a0. [DOI] [PubMed] [Google Scholar]
- Melvold RW Kohn H. I. Histocompatibility gene mutation rates: H-2 and non-H-2. Mutat Res. 1975 Mar;27(3):415–418. doi: 10.1016/0027-5107(75)90300-0. [DOI] [PubMed] [Google Scholar]
- Meruelo D. A role for elevated H-2 antigen expression in resistance to neoplasia caused by radiation-induced leukemia virus. Enhancement of effective tumor surveillance by killer lymphocytes. J Exp Med. 1979 Apr 1;149(4):898–909. doi: 10.1084/jem.149.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D. H-2D control of leukaemia susceptibility: mechanism and implications. J Immunogenet. 1980 Feb;7(1):81–90. doi: 10.1111/j.1744-313x.1980.tb00710.x. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Kramer J. H-2D control of radiation leukemia virus induced neoplasia: evidence for interaction of viral and H-2 genomic information. Transplant Proc. 1981 Dec;13(4):1858–1862. [PubMed] [Google Scholar]
- Meruelo D., McDevitt H. O. Recent studies on the role of the immune response in resistance to virus-induced leukemias and lymphomas. Semin Hematol. 1978 Oct;15(4):399–419. [PubMed] [Google Scholar]
- Meruelo D., Nimelstein S. H., Jones P. P., Lieberman M., McDevitt H. O. Increased synthesis and expression of H-2 antigens on thymocytes as a result of radiation leukemia virus infection: a possible mechanism for H-2 linked control of virus-induced neoplasia. J Exp Med. 1978 Feb 1;147(2):470–487. doi: 10.1084/jem.147.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Rossomando A., Offer M., Buxbaum J., Pellicer A. Association of endogenous viral loci with genes encoding murine histocompatibility and lymphocyte differentiation antigens. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5032–5036. doi: 10.1073/pnas.80.16.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Wallace R. B. The complete amino acid sequence of the murine transplantation antigen H-2Db as deduced by molecular cloning. Immunogenetics. 1982;16(1):1–9. doi: 10.1007/BF00364437. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S., Passmore H. C., Klein J. Genetic organization and evolution of the mouse H-2 region: a duplication model. Transplant Proc. 1971 Mar;3(1):176–179. [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Snell G. D. The H-2 locus of the mouse: observations and speculations concerning its comparative genetics and its polymorphism. Folia Biol (Praha) 1968;14(5):335–358. [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Stockert E., Old L. J., Boyse E. A. The G-IX system. A cell surface allo-antigen associated with murine leukemia virus; implications regarding chromosomal integration of the viral genome. J Exp Med. 1971 Jun 1;133(6):1334–1355. doi: 10.1084/jem.133.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]