Abstract
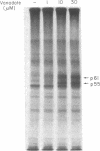
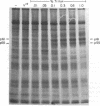
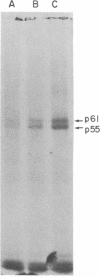
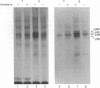
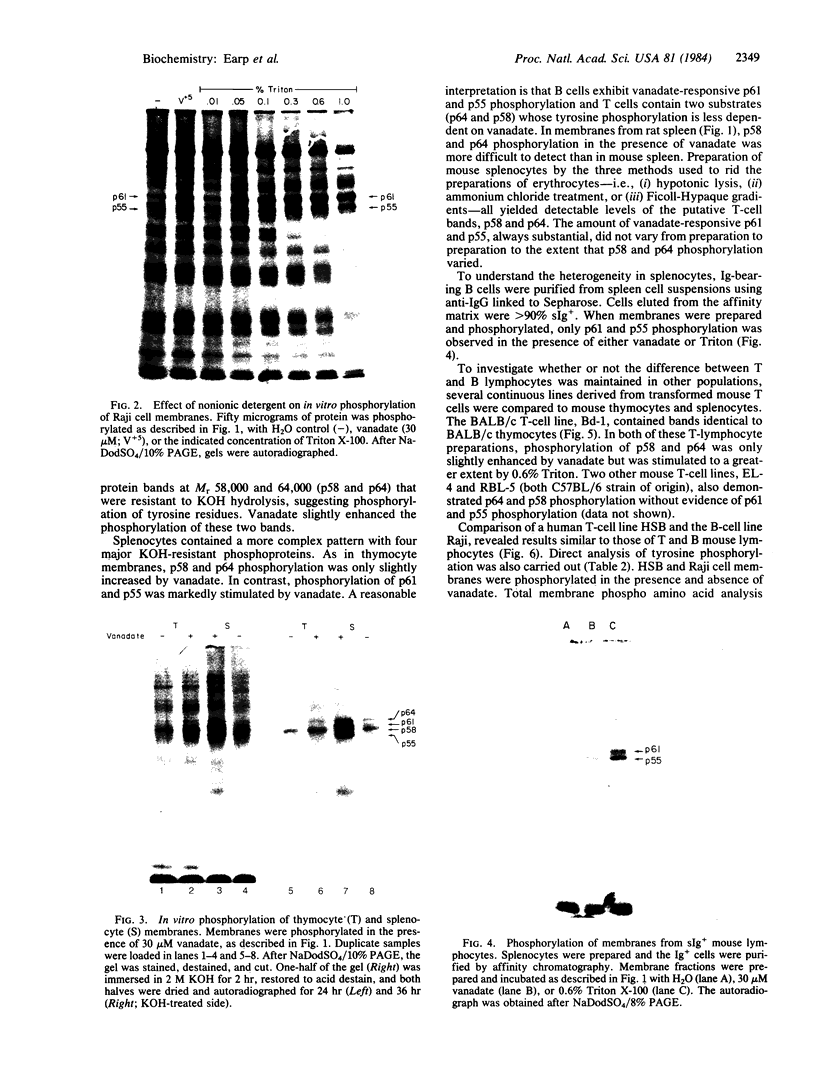
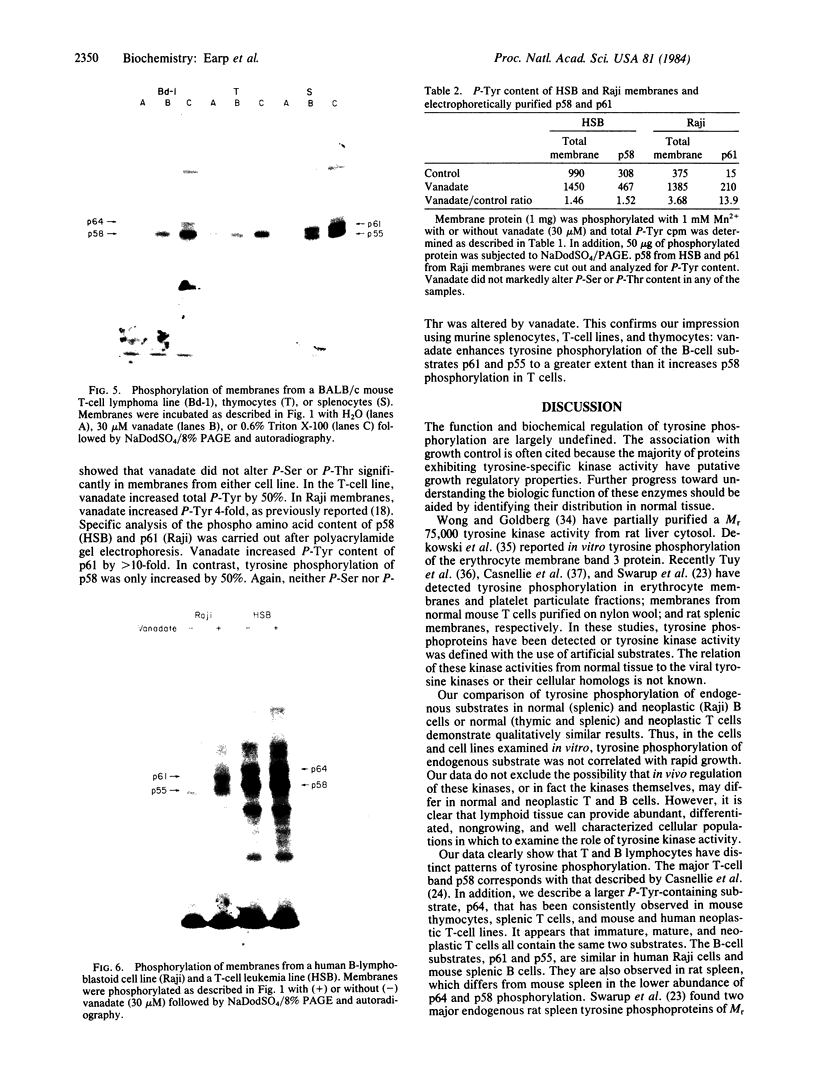
Membrane fractions isolated from mouse and rat spleen expressed substantial tyrosine-specific protein kinase activity. Phosphotyrosine (P-Tyr) accumulation in endogenous membrane substrates was stimulated by vanadate or nonionic detergents. When in vitro phosphorylation was carried out at 0 degree C in the presence of 1 mM Mn2+ and Triton X-100, P-Tyr constituted up to 40-50% of the total phospho amino acid. Polyacrylamide gel electrophoresis showed that membranes from mixed lymphocyte populations have four major P-Tyr-containing proteins. Whereas nonionic detergents were potent stimuli for P-Tyr accumulation in all four substrates, tyrosine phosphorylation of two of these (p61 and p55) was markedly dependent on vanadate. These two substrates were present in membranes from surface Ig-bearing splenic lymphocytes purified by affinity chromatography and Raji, a human B-lymphoblastoid cell line. P-Tyr accumulation in the two other substrates observed in splenocyte membranes (p64 and p58) was much less dependent on vanadate. p64 and p58 were phosphorylated in membranes from mouse thymocytes and human and mouse T-lymphoma cell lines, while p61 and p55 were not. Thus it appears that in both murine and human lymphocytes, p64 and p58 served as T-cell-specific substrates, while p61 and p55 were specifically associated with B lymphocytes. Moreover, these distinct P-Tyr substrate patterns were conserved in some neoplastic cell lines derived from B and T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978 Nov 23;276(5686):409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma cell line expressing elevated levels of tyrosine protein kinase activity. J Biol Chem. 1983 Sep 10;258(17):10738–10742. [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta J. D., Garbers D. L. Tyrosine protein kinase activity during embryogenesis. J Biol Chem. 1983 May 25;258(10):6174–6178. [PubMed] [Google Scholar]
- Dekowski S. A., Rybicki A., Drickamer K. A tyrosine kinase associated with the red cell membrane phosphorylates band 3. J Biol Chem. 1983 Mar 10;258(5):2750–2753. [PubMed] [Google Scholar]
- Earp H. S., Rubin R. A., Austin K. S., Dy R. C. DMSO increases tyrosine residue phosphorylation in membranes from murine erythroleukemia cells. Biochem Biophys Res Commun. 1983 Apr 29;112(2):413–418. doi: 10.1016/0006-291x(83)91479-1. [DOI] [PubMed] [Google Scholar]
- Earp H. S., Rubin R. A., Austin K. S., Dy R. C. Vanadate stimulates tyrosine phosphorylation of two proteins in Raji human lymphoblastoid cell membranes. FEBS Lett. 1983 Sep 19;161(2):180–184. doi: 10.1016/0014-5793(83)81003-5. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie G. Y., Hansen C. B., Hoskins R. G., Russell S. W. Inflammatory cells in solid murine neoplasms. IV. Cytolytic T lymphocytes isolated from regressing or progressing Moloney sarcomas. J Immunol. 1977 Aug;119(2):564–570. [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Law L. W., Apella E. Immunogenic properties of solubilized tumour antigen from an RNA virus-transformed neoplasm. Nature. 1973 May 11;243(5402):83–87. doi: 10.1038/243083a0. [DOI] [PubMed] [Google Scholar]
- Leis J. F., Kaplan N. O. An acid phosphatase in the plasma membranes of human astrocytoma showing marked specificity toward phosphotyrosine protein. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6507–6511. doi: 10.1073/pnas.79.21.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Engeser H., Greenberg M. E., O'Farrell M., Gall W. E., Edelman G. M. Activities of the src-gene product of avian sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):949–958. doi: 10.1101/sqb.1980.044.01.102. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Peters C. J., Theofilopoulos A. N. Antibody-dependent cellular cytotoxicity against murine leukemia viral antigens: studies with human lymphoblastoid cell lines and human peripheral lymphocytes as effector cells comparing rabbit, goat, and mouse antisera. J Immunol. 1977 Sep;119(3):1089–1096. [PubMed] [Google Scholar]
- Ramasarma T., Crane F. L. Does vanadium play a role in cellular regulation? Curr Top Cell Regul. 1981;20:247–301. doi: 10.1016/b978-0-12-152820-1.50011-0. [DOI] [PubMed] [Google Scholar]
- Richert N., Davies P. J., Jay G., Pastan I. Inhibition of the transformation-specific kinase in ASV-transformed cells by N-alpha-tosyl-L-lysyl chloromethyl ketone. Cell. 1979 Oct;18(2):369–374. doi: 10.1016/0092-8674(79)90056-4. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., Earp H. S. Dimethyl sulfoxide stimulates tyrosine residue phosphorylation of rat liver epidermal growth factor receptor. Science. 1983 Jan 7;219(4580):60–63. doi: 10.1126/science.6294827. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., Earp H. S. Solubilization of EGF receptor with Triton X-100 alters stimulation of tyrosine residue phosphorylation by EGF and dimethyl sulfoxide. J Biol Chem. 1983 Apr 25;258(8):5177–5182. [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., Hansen C. B., Cochrane C. G. Inflammatory cells in solid murine neoplasms. II. Cell types found throughout the course of Moloney sarcoma regression or progression. Int J Cancer. 1976 Sep 15;18(3):331–338. doi: 10.1002/ijc.2910180310. [DOI] [PubMed] [Google Scholar]
- Sallan S. E., Weinstein H. J., Nathan D. G. The childhood leukemias. J Pediatr. 1981 Nov;99(5):676–688. doi: 10.1016/s0022-3476(81)80384-8. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Dasgupta J. D., Garbers D. L. Tyrosine protein kinase activity of rat spleen and other tissues. J Biol Chem. 1983 Sep 10;258(17):10341–10347. [PubMed] [Google Scholar]
- Tuy F. P., Henry J., Rosenfeld C., Kahn A. High tyrosine kinase activity in normal nonproliferating cells. 1983 Sep 29-Oct 5Nature. 305(5933):435–438. doi: 10.1038/305435a0. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Tyrosyl protein kinases in normal rat liver: identification and partial characterization. Proc Natl Acad Sci U S A. 1983 May;80(9):2529–2533. doi: 10.1073/pnas.80.9.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]