Abstract
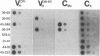
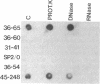
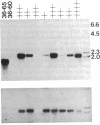
A technique is described that allows single hybridoma cell colonies to be assayed for the productive rearrangement of a single immunoglobulin variable region (V) gene segment by utilizing expression of V mRNA for analysis. Hybridomas growing in microwell tissue culture plates are lysed in situ, cellular RNA is directly transferred to nitrocellulose by filtration, and specific immunoglobulin mRNA is detected by hybridization of the filter with a DNA probe. The method is simple and sensitive. A single species of mRNA can be detected in a lysate of 1000 cells; 5000 hybridoma colonies can be easily screened per day. The technique has been successfully used to isolate cell lines from nonimmune mice expressing a particular heavy chain variable region (VH) gene segment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Lernhardt W., Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell. 1977 Jan;10(1):27–34. doi: 10.1016/0092-8674(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. K., Chang J. Y., Alkan S. S., Braun D. G. The complete amino acid sequence of the light chain variable region of two monoclonal anti-p-azobenzene-arsonate antibodies bearing the cross-reactive idiotype. Mol Immunol. 1983 Feb;20(2):197–201. doi: 10.1016/0161-5890(83)90131-1. [DOI] [PubMed] [Google Scholar]
- Bresser J., Hubbell H. R., Gillespie D. Biological activity of mRNA immobilized on nitrocellulose in NaI. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6523–6527. doi: 10.1073/pnas.80.21.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K., Tregear G. W. Solid-phase radioimmunoassay in antibody-coated tubes. Science. 1967 Dec 22;158(3808):1570–1572. doi: 10.1126/science.158.3808.1570. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Coutinho A., Melchers F. Absolute frequencies of lipopolysaccharide-reactive B cells producing A5A idiotype in unprimed, streptococcal A carbohydrate-primed, anti-A5A idiotype-sensitized and anti-A5A idiotype-suppressed A/J mice. J Exp Med. 1977 Nov 1;146(5):1436–1449. doi: 10.1084/jem.146.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foung S. K., Sasaki D. T., Grumet F. C., Engleman E. G. Production of functional human T-T hybridomas in selection medium lacking aminopterin and thymidine. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7484–7488. doi: 10.1073/pnas.79.23.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Pawlak L. L., Nisonoff A. Nature of anti-hapten antibodies arising after immune suppression of a set of cross-raactive idiotypic specificities. Eur J Immunol. 1973 Jan;3(1):44–48. doi: 10.1002/eji.1830030110. [DOI] [PubMed] [Google Scholar]
- Hornbeck P. V., Lewis G. K. Idiotype connectance in the immune system. I. Expression of a cross-reactive idiotype on induced anti-p-azophenylarsonate antibodies and on endogenous antibodies not specific for arsonate. J Exp Med. 1983 Apr 1;157(4):1116–1136. doi: 10.1084/jem.157.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Juy D., Primi D., Sanchez P., Cazenave P. A. The selection and maintenance of the V region determinant repertoire is germ-line encoded and T cell-independent. Eur J Immunol. 1983 Apr;13(4):326–331. doi: 10.1002/eji.1830130410. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Pickard A. R., Sigal N. H., Gearhart P. J., Metcalf E. S., Pierce S. K. Assessing B cell diversification by antigen receptor and precursor cell analysis. Ann Immunol (Paris) 1976 Jun-Jul;127(3-4):489–502. [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolies M. N., Marshak-Rothstein A., Gefter M. L. Structural diversity among anti-p-azophenylarsonate monoclonal antibodies from A/J mice; comparison of Id- and Id+ sequences. Mol Immunol. 1981 Dec;18(12):1065–1077. doi: 10.1016/0161-5890(81)90022-5. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Wysocki L. J., Sato V. L. Immunoglobulin idiotype and anti-anti-idiotype utilize the same variable region genes irrespective of antigen specificity. J Immunol. 1983 Feb;130(2):515–517. [PubMed] [Google Scholar]
- Nishikawa S., Takemori T., Rajewsky K. The expression of a set of antibody variable regions in lipopolysaccharide-reactive B cells at various stages of ontogeny and its control by anti-idiotypic antibody. Eur J Immunol. 1983 Apr;13(4):318–325. doi: 10.1002/eji.1830130409. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Seidman J. G., Leder P., Tonegawa S., Matthyssens G., Weigert M. Transcription of mouse kappa chain genes: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Takemori T. Genetics, expression, and function of idiotypes. Annu Rev Immunol. 1983;1:569–607. doi: 10.1146/annurev.iy.01.040183.003033. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Siegelman M., Capra J. D. Complete amino acid sequence of light chain variable regions derived from five monoclonal anti-p-azophenylarsonate antibodies differing with respect to a crossreactive idiotype. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7679–7683. doi: 10.1073/pnas.78.12.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Gefter M. L., Brodeur P., Riblet R., Marshak-Rothstein A. The genetic basis of antibody production: the dominant anti-arsonate idiotype response of the strain A mouse. Eur J Immunol. 1982 Dec;12(12):1023–1032. doi: 10.1002/eji.1830121208. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland R., Cantor H. Idiotype-specific T helper cells are required to induce idiotype-positive B memory cells to secrete antibody. Eur J Immunol. 1978 Aug;8(8):600–606. doi: 10.1002/eji.1830080812. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. The strain A anti-p-azophenylarsonate major cross-reactive idiotypic family includes members with no reactivity toward p-azophenylarsonate. Eur J Immunol. 1981 Oct;11(10):832–839. doi: 10.1002/eji.1830111016. [DOI] [PubMed] [Google Scholar]