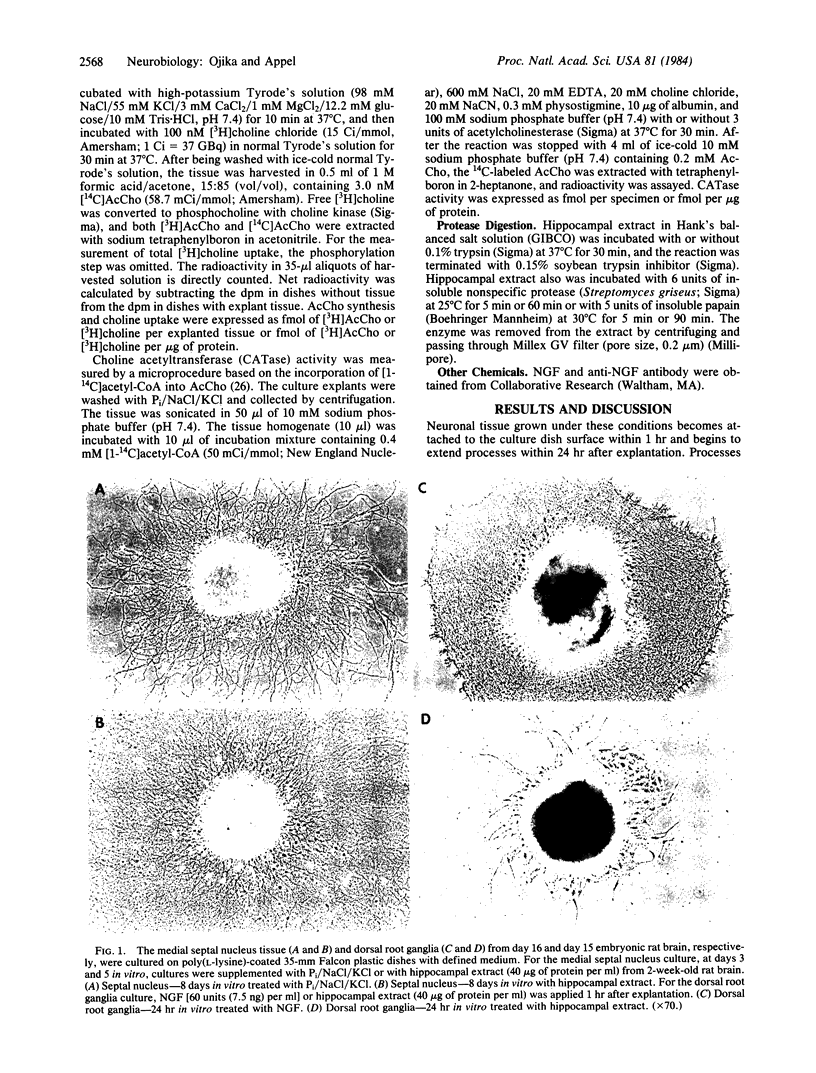
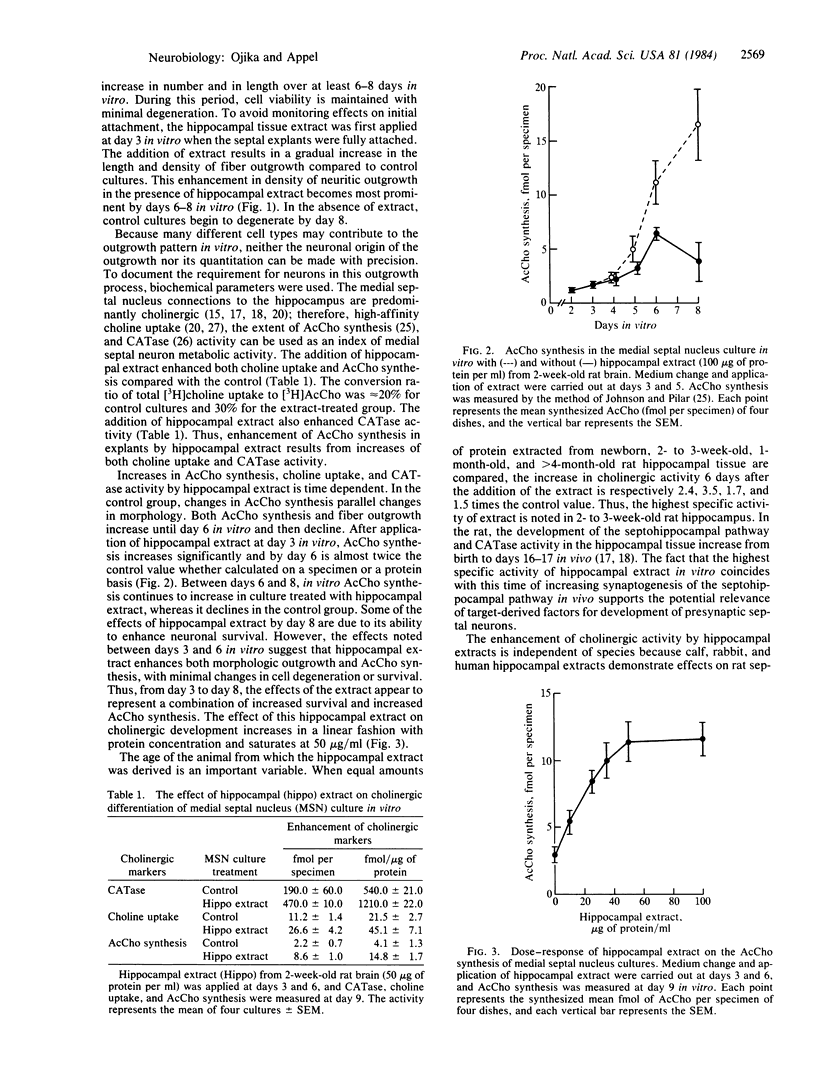
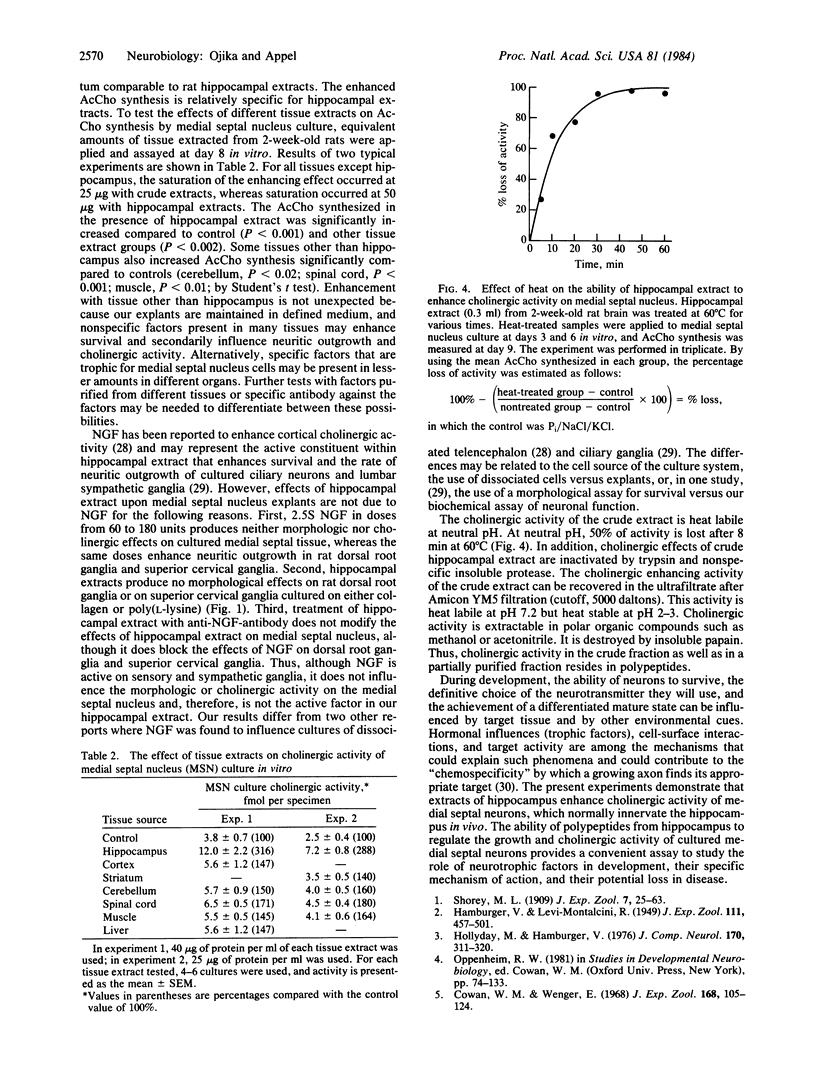
Abstract
Within peripheral sympathetic and sensory systems, target tissue provides diffusible factors such as nerve growth factor that influence neuronal survival, growth, and differentiation. To determine whether target tissue may exert retrograde effects within the central nervous system, we have used hippocampus as a source of neurotrophic factors and cultured medial septal nucleus explants as a source of neurons that may respond to such factors. Soluble extracts from rat hippocampus enhance cholinergic activity (choline acetyltransferase, choline uptake, acetylcholine synthesis) of the rat medial septal nucleus cultured in serum-free defined medium. The enhancement is dose dependent, relatively specific for hippocampus, and varies with age, reaching a peak in 2- to 3-week-old rat hippocampus. The enhancing activity of the hippocampal extract appears to be mediated by protease-sensitive polypeptide(s) that differ from nerve growth factor in biological and chemical characteristics.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel S. H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981 Dec;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Bayer S. A. The development of the septal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1979 Jan 1;183(1):89–106. doi: 10.1002/cne.901830108. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Regeneration of monoaminergic and cholinergic neurons in the mammalian central nervous system. Physiol Rev. 1979 Jan;59(1):62–100. doi: 10.1152/physrev.1979.59.1.62. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan W. M., Wenger E. Degeneration in the nucleus of origin of the preganglionic fibers to the chick ciliary ganglion following early removal of the optic vesicle. J Exp Zool. 1968 May;168(1):105–123. doi: 10.1002/jez.1401680109. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Collins F. In vitro Evidence for two distinct hippocampal growth factors: basis of neuronal plasticity? Science. 1982 Jul 2;217(4554):67–68. doi: 10.1126/science.7089542. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969 Nov;115(3):465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller E. L., Jr, Schrier B. K., Shainberg A., Fisk H. R., Nelson P. G. Choline acetyltransferase activity is increased in combined cultures of spinal cord and muscle cells from mice. Science. 1973 Nov 9;182(4112):588–589. doi: 10.1126/science.182.4112.588. [DOI] [PubMed] [Google Scholar]
- Godfrey E. W., Schrier B. K., Nelson P. G. Source and target cell specificities of a conditioned medium factor that increases choline acetyltransferase activity in cultured spinal cord cells. Dev Biol. 1980 Jun 15;77(2):403–418. doi: 10.1016/0012-1606(80)90484-4. [DOI] [PubMed] [Google Scholar]
- Hollyday M., Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976 Dec 1;170(3):311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- Honegger P., Lenoir D. Nerve growth factor (NGF) stimulation of cholinergic telencephalic neurons in aggregating cell cultures. Brain Res. 1982 Feb;255(2):229–238. doi: 10.1016/0165-3806(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Pilar G. The release of acetylcholine from post-ganglionic cell bodies in response to depolarization. J Physiol. 1980 Feb;299:605–619. doi: 10.1113/jphysiol.1980.sp013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar M. J., Sethy V. H., Roth R. H., Aghajanian G. K. Choline: selective accumulation by central cholinergic neurons. J Neurochem. 1973 Feb;20(2):581–593. doi: 10.1111/j.1471-4159.1973.tb12157.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967 Sep;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Skaper S., Adler R., Landa K., Varon S. Cholinergic neuronotrophic factors: fractionation properties of an extract from selected chick embryonic eye tissues. J Neurochem. 1980 Jan;34(1):69–75. doi: 10.1111/j.1471-4159.1980.tb04622.x. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Nadler J. V., Lynch G. S., Cotman C. W. Development of cholinergic innervation in the hippocampal formation of the rat. I. Histochemical demonstration of acetylcholinesterase activity. Dev Biol. 1974 Jan;36(1):130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- Nadler J. V., Matthews D. A., Cotman C. W., Lynch G. S. Development of cholinergic innervation in the hippocampal formation of the rat. II. Quantitative changes in choline acetyltransferase and acetylcholinesterase activities. Dev Biol. 1974 Jan;36(1):142–154. doi: 10.1016/0012-1606(74)90197-3. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld A. R., Thal L. J., Horowitz S. G., Katzman R. Heart conditioned medium promotes central cholinergic regeneration in vivo. Brain Res. 1981 Dec 21;229(2):541–546. doi: 10.1016/0006-8993(81)91019-2. [DOI] [PubMed] [Google Scholar]
- Segal M., Landis S. Afferents to the hippocampus of the rat studied with the method of retrograde transport of horseradish peroxidase. Brain Res. 1974 Sep 20;78(1):1–15. doi: 10.1016/0006-8993(74)90349-7. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Choline: high-affinity uptake by rat brain synaptosomes. Science. 1972 Nov 10;178(4061):626–628. doi: 10.1126/science.178.4061.626. [DOI] [PubMed] [Google Scholar]