Abstract
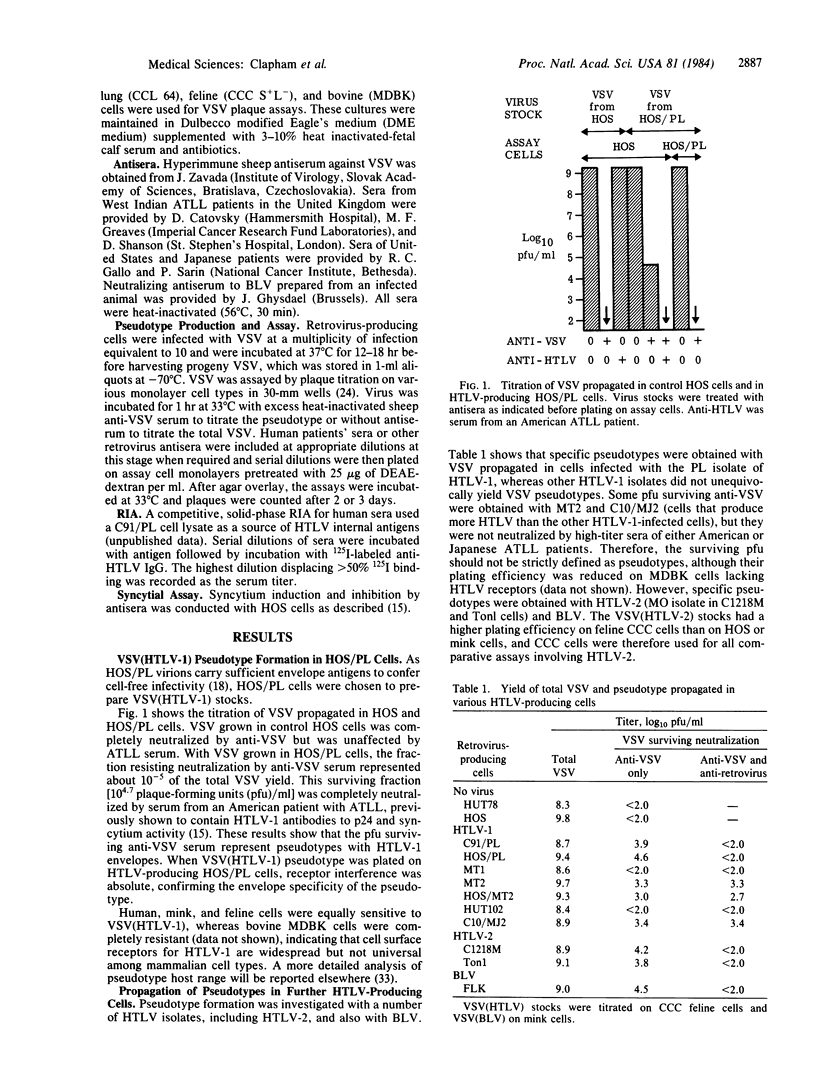
Pseudotypes of vesicular stomatitis virus (VSV) bearing envelope antigens of human T-cell leukemia virus (HTLV) types 1 and 2 were prepared by propagating VSV in cells lines productively infected with HTLV. Plaque assays of VSV (HTLV) pseudotypes were employed to determine the presence of (i) HTLV receptors on cells and (ii) neutralizing antibodies in the serum of patients with adult T-cell leukemia-lymphoma (ATLL). Cell surface receptors for HTLV-1 and HTLV-2 were found on nonlymphoid cells of human and mammalian origin. Neutralizing antibodies specific to VSV(HTLV-1) were found in sera of ATLL patients in titers varying from 1:50 to 1:30,000 and did not correlate closely with antibody titers for internal viral antigens. Sera from ATLL patients in the United Kingdom (Caribbean immigrants), United States, and Japan completely neutralized VSV (HTLV-1), indicating that the HTLV isolates from these distinct geographic regions represent a single envelope serotype. Neutralization of VSV (HTLV-1) was more specific and more sensitive than assays of syncytium inhibition. No cross-neutralization was observed between bovine leukosis virus and HTLV, and only limited cross-reaction was found for envelope antigens of HTLV-1 and HTLV-2. These studies show that VSV (HTLV) pseudotypes can be readily used to screen for neutralizing antibodies in patients' sera and to distinguish HTLV envelope serotypes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner W. A., Gibbs W. N., Saxinger C., Robert-Guroff M., Clark J., Lofters W., Hanchard B., Campbell M., Gallo R. C. Human T-cell leukaemia/lymphoma virus-associated lymphoreticular neoplasia in Jamaica. Lancet. 1983 Jul 9;2(8341):61–64. doi: 10.1016/s0140-6736(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Love D. N., Weiss R. A. Virus envelope markers in mammalian tropism of avian RNA tumor viruses. J Virol. 1975 Jan;15(1):108–114. doi: 10.1128/jvi.15.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catovsky D., Greaves M. F., Rose M., Galton D. A., Goolden A. W., McCluskey D. R., White J. M., Lampert I., Bourikas G., Ireland R. Adult T-cell lymphoma-leukaemia in Blacks from the West Indies. Lancet. 1982 Mar 20;1(8273):639–643. doi: 10.1016/s0140-6736(82)92200-0. [DOI] [PubMed] [Google Scholar]
- Chan J. C., East J. L., Bowen J. M., Massey R., Schochetman G. Monoclonal and polyclonal antibody studies of VSV(hrMMTV) pseudotypes. Virology. 1982 Jul 15;120(1):54–64. doi: 10.1016/0042-6822(82)90006-x. [DOI] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H., Shimoyama M., Miwa M., Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Howard T., Spence C. Identification of infectious virions which are vesicular stomatitis virus pseudotypes of murine type C virus. Virology. 1976 Apr;70(2):432–439. doi: 10.1016/0042-6822(76)90284-1. [DOI] [PubMed] [Google Scholar]
- Love D. N., Weiss R. A. Pseudotypes of vesicular stomatitis virus determined by exogenous and endogenous avian RNA tumor viruses. Virology. 1974 Jan;57(1):271–278. doi: 10.1016/0042-6822(74)90127-5. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Ohtsuki Y., Fujishita M., Yoshimoto S., Kubonishi I., Minezawa M. Detection of type C virus particles in Japanese monkeys seropositive to adult T-cell leukemia-associated antigens. Gan. 1982 Dec;73(6):848–849. [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Sarngadharan M. G., Copeland T. D., Kalyanaraman V. S., Gilden R. V., Gallo R. C. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Reitz M. S., Jr, Sarngadharan M. G., Robert-Guroff M., Kalyanaraman V. S., Nakao Y., Miyoshi I., Minowada J., Yoshida M., Ito Y. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982 Nov 4;300(5887):63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Kalyanaraman V. S., Sarngadharan M. G., Nakao Y., Ito Y., Gallo R. C. Antibodies against three purified structural proteins of the human type-C retrovirus, HTLV, in Japanese adult T-cell leukemia patients, healthy family members, and unrelated normals. Int J Cancer. 1983 Nov 15;32(5):583–590. doi: 10.1002/ijc.2910320511. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Yoshida M. Human adult T-cell leukemia virus: molecular cloning of the provirus DNA and the unique terminal structure. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6899–6902. doi: 10.1073/pnas.79.22.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiry L., Sprecher-Goldberger S., Bossens M., Cogniaux-Le Clerc J., Vereerstraeten P. Neutralization of Mason-Pfizer virus by sera from patients treated for renal disease. J Gen Virol. 1978 Dec;41(3):587–597. doi: 10.1099/0022-1317-41-3-587. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Watanabe T., Seiki M., Yoshida M. Retrovirus terminology. Science. 1983 Dec 16;222(4629):1178–1178. doi: 10.1126/science.6316504. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Bennett P. L. Assembly of membrane glycoproteins studied by phenotypic mixing between mutants of vesicular stomatitis virus and retroviruses. Virology. 1980 Jan 30;100(2):252–274. doi: 10.1016/0042-6822(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Boettiger D., Murphy H. M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977 Feb;76(2):808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Hinuma Y., zur Hausen H., Schneider J., Hunsmann G. African green monkeys are infected with adult T-cell leukaemia virus or closely related agent. Lancet. 1983 Jan 29;1(8318):240–241. doi: 10.1016/s0140-6736(83)92614-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada J., Cerný L., Závadová Z., Bozonová J., Altstein A. D. A rapid neutralization test for antibodies to bovine leukemia virus, with the use of rhabdovirus pseudotypes. J Natl Cancer Inst. 1979 Jan;62(1):95–101. [PubMed] [Google Scholar]
- Závada J., Dickson C., Weiss R. Pseudotypes of vesicular stomatitis virus with envelope antigens provided by murine mammary tumor virus. Virology. 1977 Oct 1;82(1):221–231. doi: 10.1016/0042-6822(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]


