Abstract
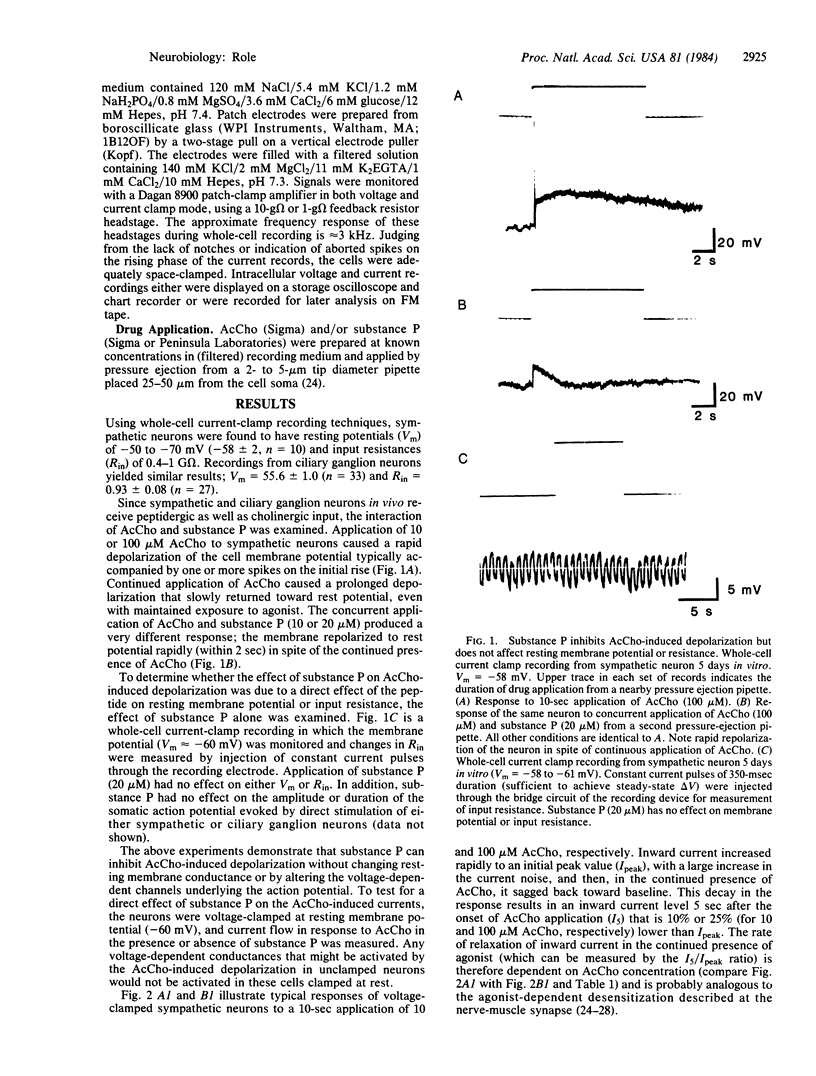
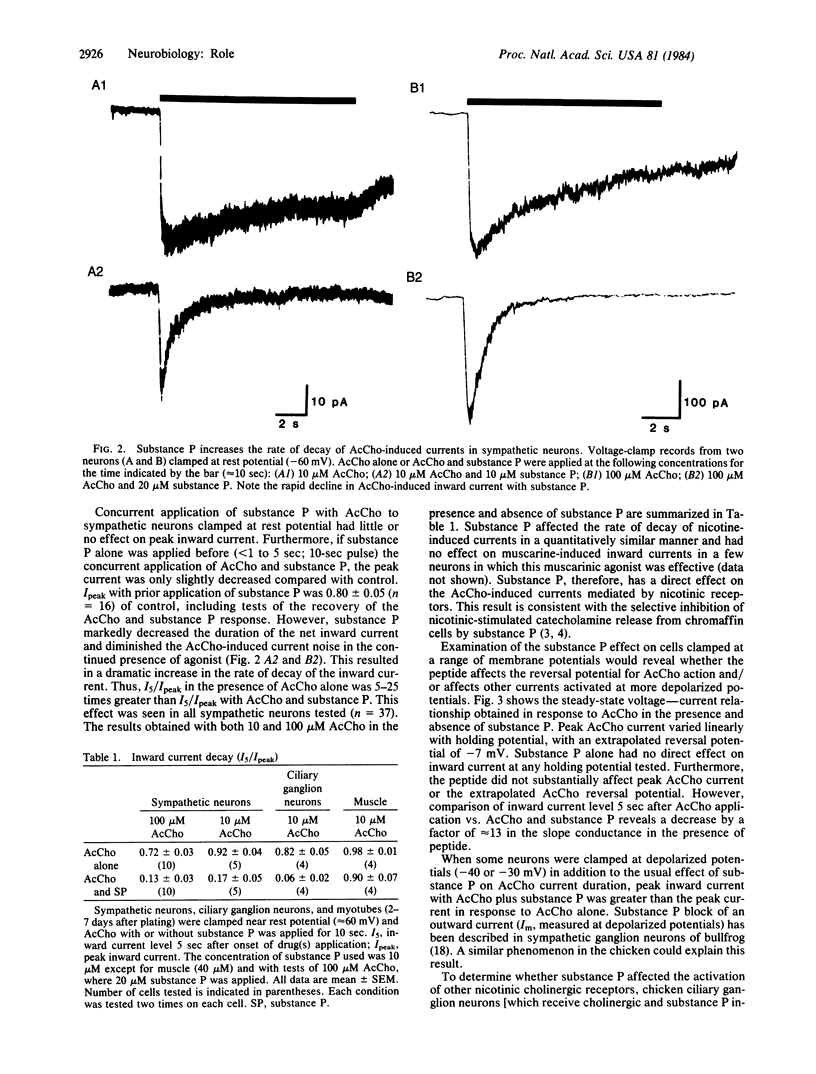
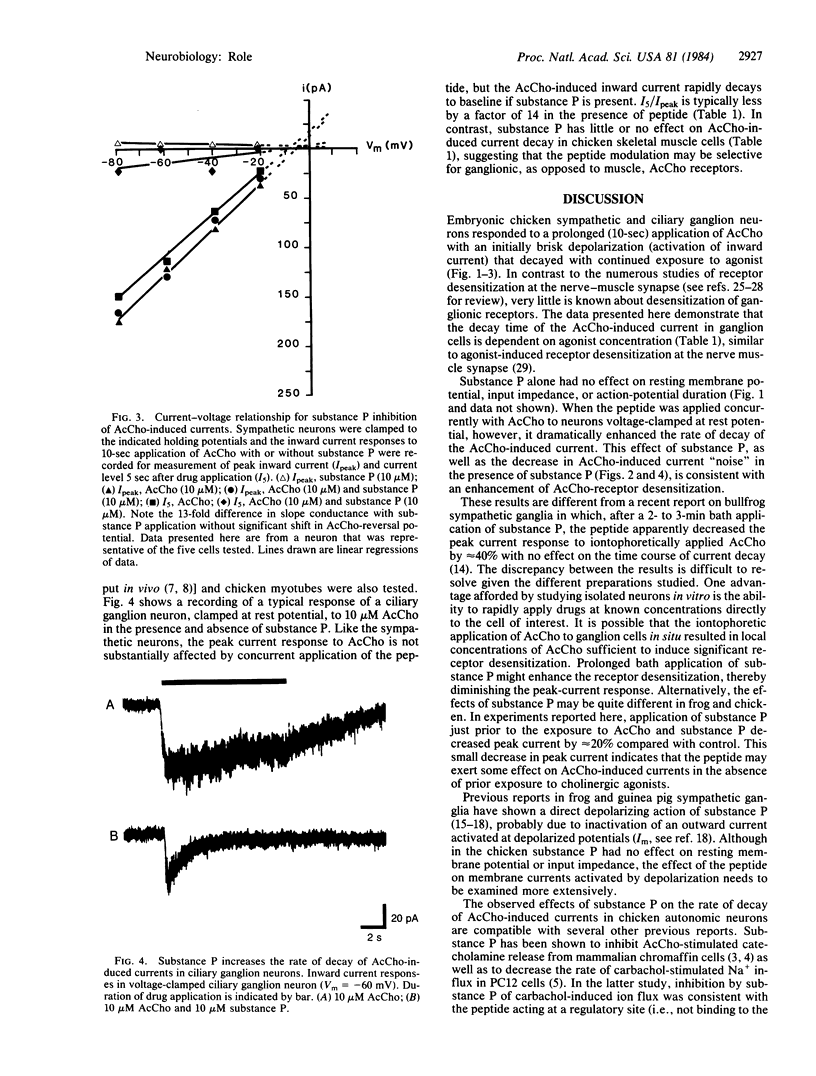
Substance P has been identified by combined immunohistochemical and radioimmunological techniques to be present in preganglionic cholinergic and sensory nerve fibers of amphibian, mammalian, and avian autonomic ganglia. The peptide has been shown to depolarize sympathetic neurons of frog and guinea pig and to decrease the cholinergic activation of Na+ influx and catecholamine release from chromaffin cells. The aim of this study was to examine the interaction of acetylcholine and substance P on autonomic neurons. This report demonstrates a direct effect of substance P on acetylcholine-induced inward currents in both sympathetic and parasympathetic neurons clamped near resting membrane potential. Under these conditions, substance P dramatically enhances the rate of decay of the inward current in the continued presence of agonist without substantially affecting peak inward current. This effect is consistent with an enhancement of acetylcholine-receptor desensitization. Since substance P-containing cell bodies have been demonstrated in the avian (preganglionic) column of Terni as well as in fibers from the nucleus of Edinger-Westphal, the observed peptide inhibition of cholinergic activation of the neurons may function physiologically to modulate synaptic function in autonomic ganglia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Jones S. W. Substance P inhibits the M-current in bullfrog sympathetic neurones. Br J Pharmacol. 1983 Jun;79(2):330–333. doi: 10.1111/j.1476-5381.1983.tb11004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Kojima M., Koketsu K. Substance P modulates the sensitivity of the nicotinic receptor in amphibian cholinergic transmission. Br J Pharmacol. 1983 Sep;80(1):123–131. doi: 10.1111/j.1476-5381.1983.tb11057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W. Substance P and Renshaw cells: a new concept of inhibitory synaptic interactions. J Physiol. 1977 Oct;272(1):105–119. doi: 10.1113/jphysiol.1977.sp012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Fischbach G. D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Clusters of acetylcholine receptors located at identified nerve-muscle synapses in vitro. Dev Biol. 1977 Aug;59(1):24–38. doi: 10.1016/0012-1606(77)90237-8. [DOI] [PubMed] [Google Scholar]
- Collins F. Axon initiation by ciliary neurons in culture. Dev Biol. 1978 Jul;65(1):50–57. doi: 10.1016/0012-1606(78)90178-1. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Elfvin L. G., Skirboll L., Emson P. Substance P-containing primary sensory neurons projecting to the inferior mesenteric ganglion: evidence from combined retrograde tracing and immunohistochemistry. Neuroscience. 1982 Mar;7(3):647–654. doi: 10.1016/0306-4522(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Minota S. Effects of substance P on neurones of the inferior mesenteric ganglia of the guinea-pig. J Physiol. 1981 Dec;321:259–271. doi: 10.1113/jphysiol.1981.sp013982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen J. T., Karten H. J., Eldred W. D., Brecha N. C. Localization of substance P-like and enkephalin-like immunoreactivity within preganglionic terminals of the avian ciliary ganglion: light and electron microscopy. J Neurosci. 1982 Jul;2(7):994–1003. doi: 10.1523/JNEUROSCI.02-07-00994.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen J. T., Reiner A., Karten H. J. Co-occurrence of substance P-like and Leu-enkephalin-like immunoreactivities in neurones and fibres of avian nervous system. Nature. 1982 Feb 4;295(5848):407–410. doi: 10.1038/295407a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elfvin L. G., Schultzberg M., Goldstein M., Nilsson G. On the occurrence of substance P-containing fibers in sympathetic ganglia: immunohistochemical evidence. Brain Res. 1977 Aug 19;132(1):29–41. doi: 10.1016/0006-8993(77)90704-1. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. M., Greene L. A. Histofluorescence study of chromaffin cells in dissociated cell cultures of chick embryo sympathetic ganglia. J Neurobiol. 1974;5(1):65–83. doi: 10.1002/neu.480050107. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J Physiol. 1982 Jun;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Generation of slow postsynaptic potentials without increases in ionic conductance. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1304–1311. doi: 10.1073/pnas.60.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J., Durant N. N., Henderson E. G. Drug-induced modification of ionic conductance at the neuromuscular junction. Annu Rev Pharmacol Toxicol. 1983;23:505–539. doi: 10.1146/annurev.pa.23.040183.002445. [DOI] [PubMed] [Google Scholar]
- Lavalley A. L., Ho R. H. Substance P, somatostatin, and methionine enkephalin immunoreactive elements in the spinal cord of the domestic fowl, Gallus domesticus. J Comp Neurol. 1983 Feb 1;213(4):406–413. doi: 10.1002/cne.902130405. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Cuello A. C. Substance P-immunoreactive peripheral branches of sensory neurons innervate guinea pig sympathetic neurons. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1668–1672. doi: 10.1073/pnas.79.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIsaac R. J. Afterdischarge on postganglionic sympathetic nerves following repetitive stimulation of the preganglionic nerve to the rat superior cervical ganglion in vitro. J Pharmacol Exp Ther. 1977 Jan;200(1):107–116. [PubMed] [Google Scholar]
- Mizobe F., Kozousek V., Dean D. M., Livett B. G. Pharmacological characterization of adrenal paraneurons: substance P and somatostatin as inhibitory modulators of the nicotinic response. Brain Res. 1979 Dec 14;178(2-3):555–566. doi: 10.1016/0006-8993(79)90714-5. [DOI] [PubMed] [Google Scholar]
- Nishi R., Berg D. K. Dissociated ciliary ganglion neurons in vitro: survival and synapse formation. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5171–5175. doi: 10.1073/pnas.74.11.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Role L. W., Leeman S. E., Perlman R. L. Somatostatin and substance P inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience. 1981;6(9):1813–1821. doi: 10.1016/0306-4522(81)90215-3. [DOI] [PubMed] [Google Scholar]
- Rougon G., Noble M., Mudge A. W. Neuropeptides modulate the beta-adrenergic response of purified astrocytes in vitro. Nature. 1983 Oct 20;305(5936):715–717. doi: 10.1038/305715a0. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Modulation of cholinergic transmission by substance P. Ciba Found Symp. 1982;(91):267–280. doi: 10.1002/9780470720738.ch15. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Stallcup W. B., Patrick J. Substance P enhances cholinergic receptor desensitization in a clonal nerve cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):634–638. doi: 10.1073/pnas.77.1.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoo A., Konishi S., Otsuka M. Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience. 1982;7(9):2025–2037. doi: 10.1016/0306-4522(82)90117-8. [DOI] [PubMed] [Google Scholar]


