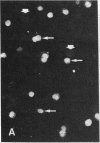
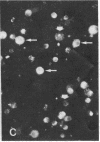
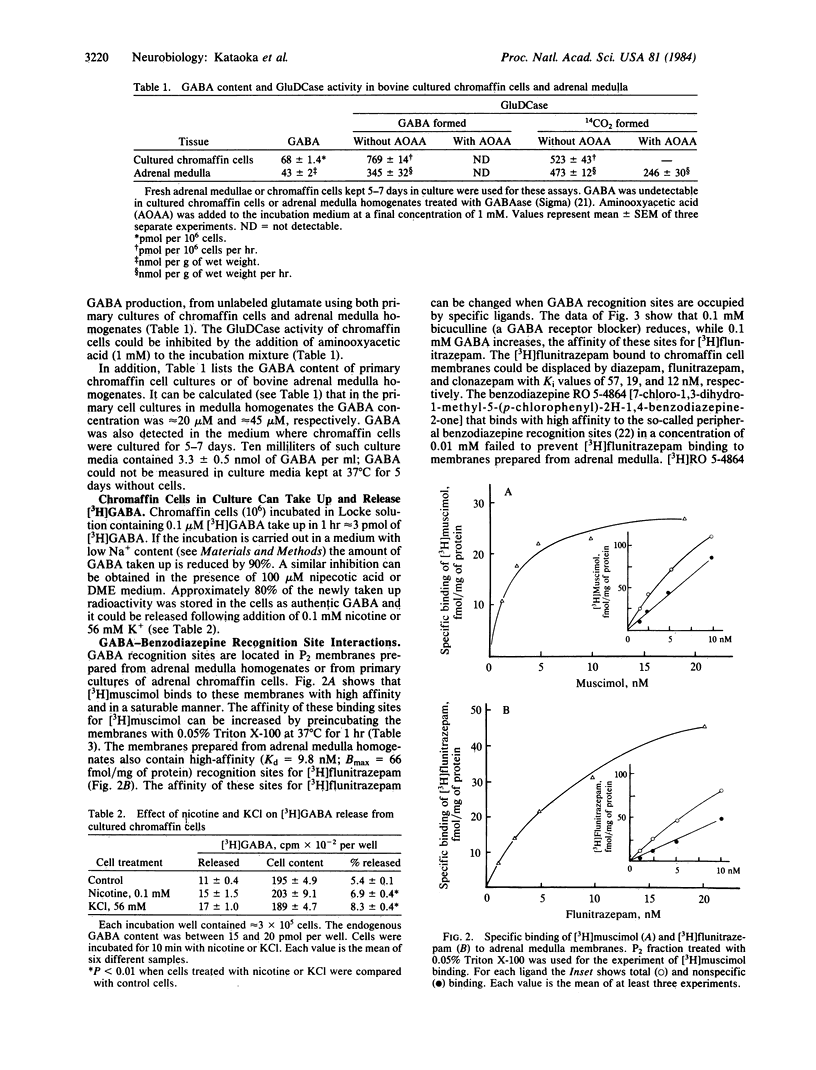
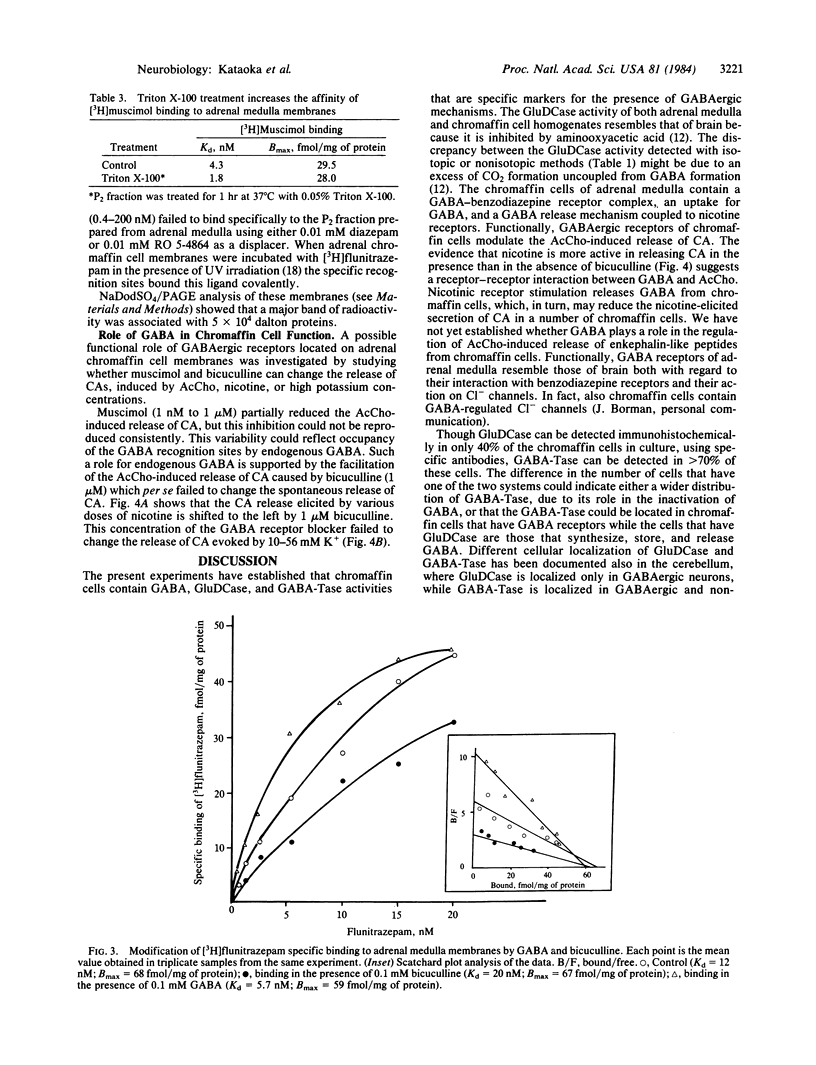
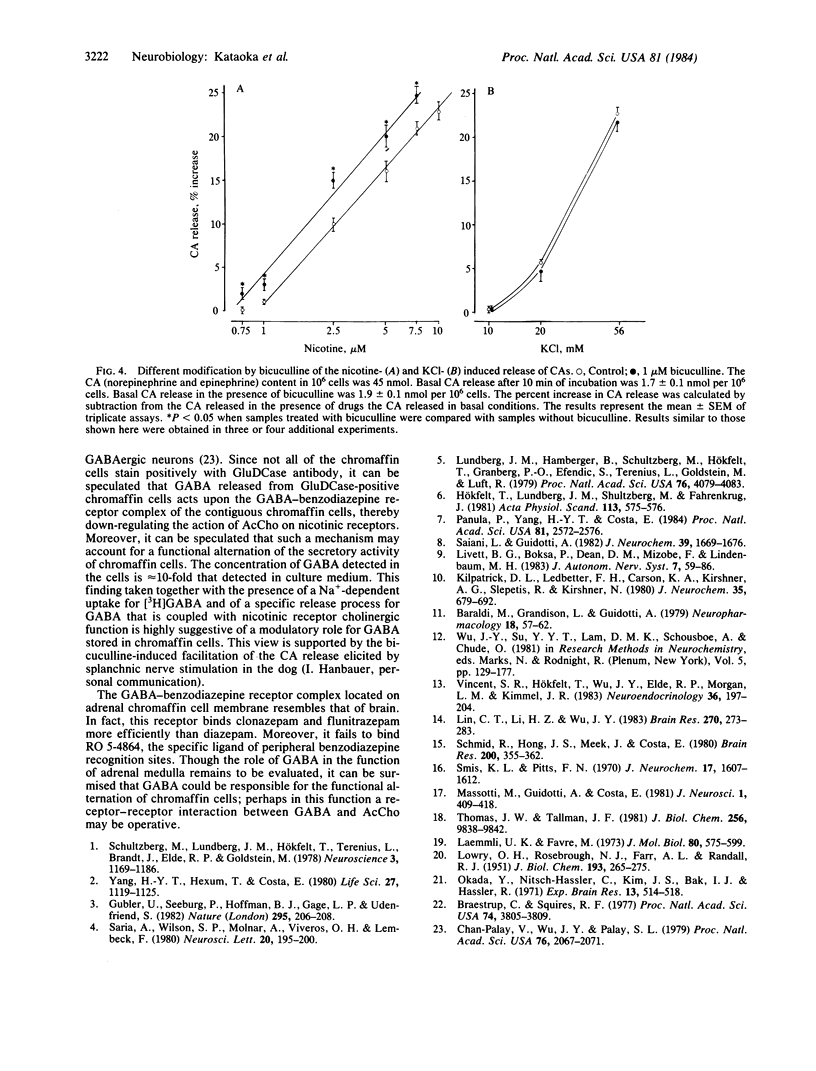
Abstract
Histochemical and biochemical studies demonstrate that gamma-aminobutyric acid (GABA), glutamic acid decarboxylase (EC 4.1.1.15), and GABA aminotransferase (EC 2.6.1.19) are present in bovine adrenal chromaffin cells. Moreover, [3H]GABA can be taken up and stored by primary cultures of adrenal chromaffin cells. Nicotinic receptor stimulation or KCl depolarization releases the [3H]GABA taken up by these cell cultures. GABA and benzodiazepine recognition sites located in chromaffin cells interact with each other with modalities similar to those described for GABA and benzodiazepine recognition sites located in synaptic membranes prepared from brain tissue. Bicuculline facilitates the release of catecholamine from chromaffin cells induced by nicotinic receptor stimulation but it fails to influence the release of catecholamine evoked by K+ depolarization. Since the GABA-benzodiazepine receptor system appears to modulate nicotinic receptor function, it is suggested that GABA transmission might participate in modulating responsiveness of chromaffin cells to incoming cholinergic stimuli.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baraldi M., Grandison L., Guidotti A. Distribution and metabolism of muscimol in the brain and other tissues of the rat. Neuropharmacology. 1979 Jan;18(1):57–62. doi: 10.1016/0028-3908(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Squires R. F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Wu J. Y., Palay S. L. Immunocytochemical localization of gamma-aminobutyric acid transaminase at cellular and ultrastructural levels. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2067–2071. doi: 10.1073/pnas.76.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Schultzberg M., Fahrenkrug J. Immunohistochemical evidence for a local VIP-ergic neuron system in the adrenal gland of the rat. Acta Physiol Scand. 1981 Dec;113(4):575–576. doi: 10.1111/j.1748-1716.1981.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Ledbetter F. H., Carson K. A., Kirshner A. G., Slepetis R., Kirshner N. Stability of bovine adrenal medulla cells in culture. J Neurochem. 1980 Sep;35(3):679–692. doi: 10.1111/j.1471-4159.1980.tb03707.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lin C. T., Li H. Z., WU J. Y. Immunocytochemical localization of L-glutamate decarboxylase, gamma-aminobutyric acid transaminase, cysteine sulfinic acid decarboxylase, aspartate aminotransferase and somatostatin in rat retina. Brain Res. 1983 Jul 4;270(2):273–283. doi: 10.1016/0006-8993(83)90601-7. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Boksa P., Dean D. M., Mizobe F., Lindenbaum M. H. Use of isolated chromaffin cells to study basic release mechanisms. J Auton Nerv Syst. 1983 Jan;7(1):59–86. doi: 10.1016/0165-1838(83)90069-3. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hamberger B., Schultzberg M., Hökfelt T., Granberg P. O., Efendić S., Terenius L., Goldstein M., Luft R. Enkephalin- and somatostatin-like immunoreactivities in human adrenal medulla and pheochromocytoma. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4079–4083. doi: 10.1073/pnas.76.8.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massotti M., Guidotti A., Costa E. Characterization of benzodiazepine and gamma-aminobutyric recognition sites and their endogenous modulators. J Neurosci. 1981 Apr;1(4):409–418. doi: 10.1523/JNEUROSCI.01-04-00409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Nitsch-Hassler C., Kim J. S., Bak I. J., Hassler R. Role of -aminobutyric acid (GABA) in the extrapyramidal motor system. 1. Regional distribution of GABA in rabbit, rat, guinea pig and baboon CNS. Exp Brain Res. 1971 Nov 30;13(5):514–518. doi: 10.1007/BF00234282. [DOI] [PubMed] [Google Scholar]
- Panula P., Yang H. Y., Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiani L., Guidotti A. Opiate receptor-mediated inhibition of catecholamine release in primary cultures of bovine adrenal chromaffin cells. J Neurochem. 1982 Dec;39(6):1669–1676. doi: 10.1111/j.1471-4159.1982.tb08001.x. [DOI] [PubMed] [Google Scholar]
- Saria A., Wilson S. P., Molnar A., Viveros O. H., Lembeck F. Substance P and opiate-like peptides in human adrenal medulla. Neurosci Lett. 1980 Nov;20(2):195–200. doi: 10.1016/0304-3940(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Schmid R., Hong J. S., Meek J., Costa E. The effect of kainic acid on the hippocampal content of putative transmitter amino acids. Brain Res. 1980 Nov 3;200(2):355–362. doi: 10.1016/0006-8993(80)90926-9. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Sims K. L., Pitts F. N., Jr Brain glutamate decarboxylase: changes in the developing rat brain. J Neurochem. 1970 Nov;17(11):1607–1612. doi: 10.1111/j.1471-4159.1970.tb03731.x. [DOI] [PubMed] [Google Scholar]
- Thomas J. W., Tallman J. F. Characterization of photoaffinity labeling of benzodiazepine binding sites. J Biol Chem. 1981 Oct 10;256(19):9838–9842. [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y., Elde R. P., Morgan L. M., Kimmel J. R. Immunohistochemical studies of the GABA system in the pancreas. Neuroendocrinology. 1983;36(3):197–204. doi: 10.1159/000123456. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Hexum T., Costa E. Opioid peptides in adrenal gland. Life Sci. 1980 Sep 29;27(13):1119–1125. doi: 10.1016/0024-3205(80)90461-0. [DOI] [PubMed] [Google Scholar]