Abstract
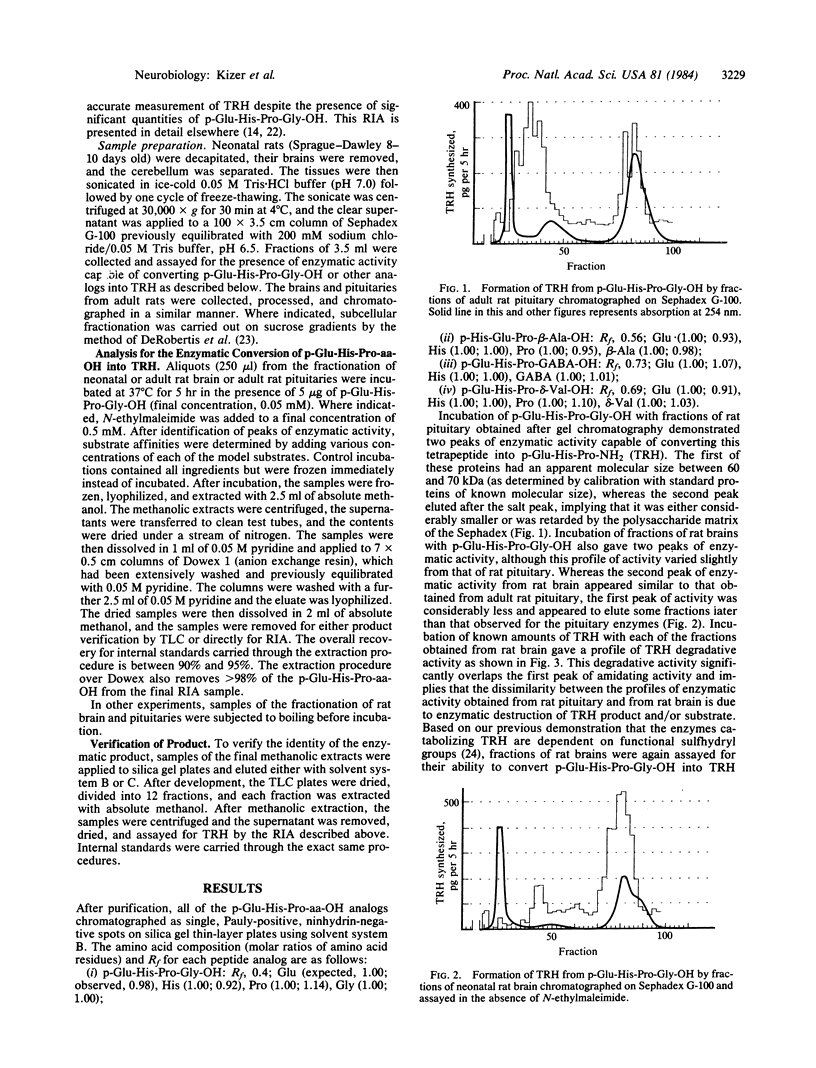
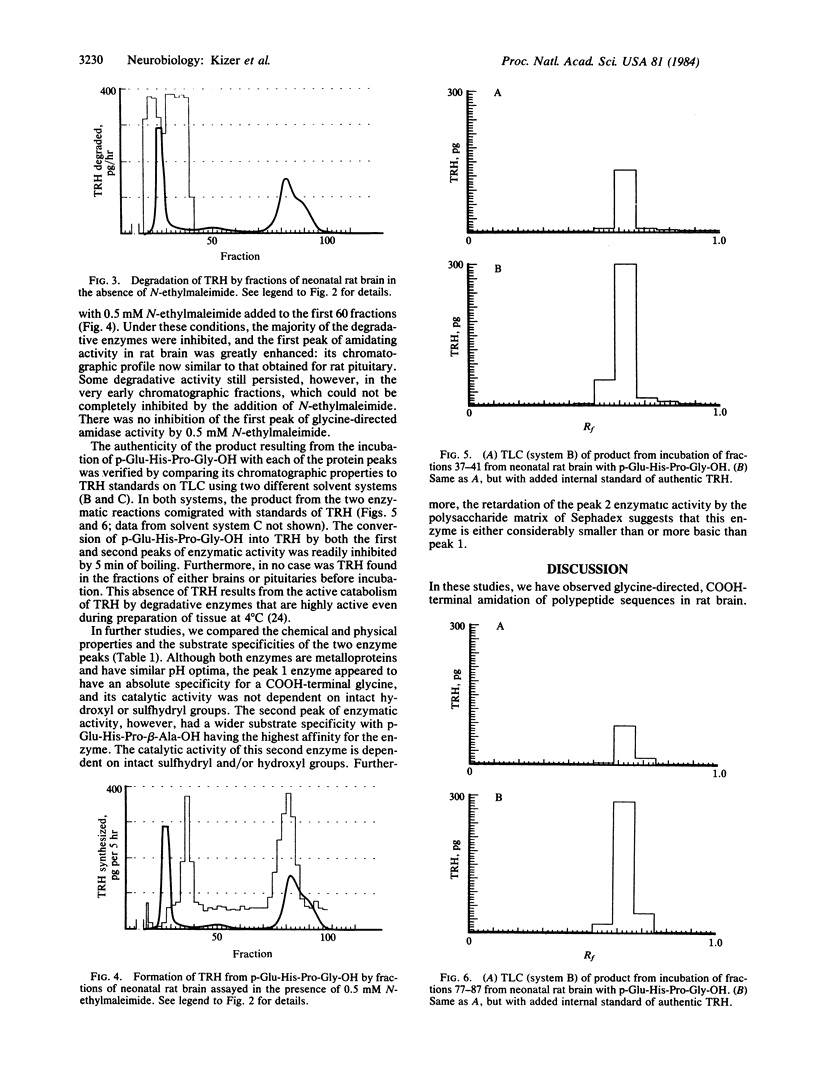
To study the possibility of glycine-directed amidation in rat brain, we synthesized the substrate p-Glu-His-Pro-Gly-OH. Adult and neonatal rat brain and adult rat pituitary were sonicated, frozen and thawed, and fractionated by gel permeation chromatography, and fractions from each tissue were assayed for enzymatic activity capable of converting this model substrate into thyrotropin-releasing hormone. We report the presence in rat brain and rat pituitary of two enzymes catalyzing conversion of p-Glu-His-Pro-Gly-OH into thyrotropin-releasing hormone. Based on the differing chemical and physical properties of these two enzymes and their differing affinities for a number of p-Glu-His-Pro-aa-OH analogs (in which aa = glycine, beta-alanine, gamma-butyric acid, and delta-aminovaleric acid), we conclude that there are two distinct enzymatic processes for the terminal amidation of peptides in brain and that COOH-terminal extensions other than glycine are capable of directing COOH-terminal amidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., David D. N., Rosenfeld M. G., Roos B. A., Evans R. M. Characterization of rat calcitonin mRNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4444–4448. doi: 10.1073/pnas.77.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara S. G., David D. N., Rosenfeld M. G., Roos B. A., Evans R. M. Characterization of rat calcitonin mRNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4444–4448. doi: 10.1073/pnas.77.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Boguslawski S., Caine J. The translation of a human placental lactogen mRNA fraction in heterologous cell-free systems: the synthesis of a possible precursor. Biochem Biophys Res Commun. 1975 Jan 6;62(1):103–109. doi: 10.1016/s0006-291x(75)80411-6. [DOI] [PubMed] [Google Scholar]
- Boler J., Chang J. K., Enzmann F., Folkers K. Synthesis of the thyrotropin-releasing hormone. J Med Chem. 1971 Jun;14(6):475–476. doi: 10.1021/jm00288a002. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Substrate specificity of an amidating enzyme in porcine pituitary. Biochem Biophys Res Commun. 1983 Apr 29;112(2):372–377. doi: 10.1016/0006-291x(83)91473-0. [DOI] [PubMed] [Google Scholar]
- Busby W. H., Jr, Youngblood W. W., Kizer J. S. Studies of substrate requirements, kinetic properties, and competive inhibitors of the enzymes catabolizing TRH in rat brain. Brain Res. 1982 Jun 24;242(2):261–270. doi: 10.1016/0006-8993(82)90309-2. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E., PELLEGRINO DE IRALDI A., RODRIGUEZ DE LORES GARNAIZ G., SALGANICOFF L. Cholinergic and non-cholinergic nerve endings in rat brain. I. Isolation and subcellular distribution of acetylcholine and acetylcholinesterase. J Neurochem. 1962 Jan-Feb;9:23–35. doi: 10.1111/j.1471-4159.1962.tb07489.x. [DOI] [PubMed] [Google Scholar]
- Fernlund P. Structure of the red-pigment-concentrating hormone of the shrimp, Pandalus borealis. Biochim Biophys Acta. 1974 Dec 18;371(2):304–311. doi: 10.1016/0005-2795(74)90027-0. [DOI] [PubMed] [Google Scholar]
- Geiger R., König W., Sandow J., Schally A. V. (8-Homoarginin)(Lutenisierendes Hormon freisetzendes Hormon) Hoppe Seylers Z Physiol Chem. 1974 Dec;355(12):1526–1534. [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr, Rich A. Pre - proparathyroid hormone identified by cell - free translation of messenger RNA from hyperplastic human parathyroid tissue. J Clin Invest. 1975 Nov;56(5):1328–1333. doi: 10.1172/JCI108210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Tate S. S. Formation of the COOH-terminal amide group of thyrotropin-releasing-factor. FEBS Lett. 1983 Feb 21;152(2):277–281. doi: 10.1016/0014-5793(83)80395-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cho S., Oyer P. E., Terris S., Peterson J. D., Rubenstein A. H. Isolation and characterization of proinsulin C-peptide from bovine pancreas. J Biol Chem. 1971 Mar 10;246(5):1365–1374. [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Stone J. V., Mordue W., Batley K. E., Morris H. R. Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature. 1976 Sep 16;263(5574):207–211. doi: 10.1038/263207a0. [DOI] [PubMed] [Google Scholar]
- Suchanek G., Kreil G. Translation of melittin messenger RNA in vitro yields a product terminating with glutaminylglycine rather than with glutaminamide. Proc Natl Acad Sci U S A. 1977 Mar;74(3):975–978. doi: 10.1073/pnas.74.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsafriri A., Lieberman M. E., Koch Y., Bauminger S., Chobsieng P., Zor U., Lindner H. R. Capacity of immunologically purified FSH to stimulate cyclic AMP accumulation and steroidogenesis in Graafian follicles and to induce ovum maturation and ovulation in the rat. Endocrinology. 1976 Mar;98(3):655–661. doi: 10.1210/endo-98-3-655. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Voelter W., Zech K. High-performance liquid chromatographic analysis of amino acids and peptide-hormone hydrolysates in the picomole range. J Chromatogr. 1975 Oct 29;112:643–649. doi: 10.1016/s0021-9673(00)99993-x. [DOI] [PubMed] [Google Scholar]
- Youngblood W. W., Kizer J. S. Strategies for the preparation of haptens for conjugation and substrates for iodination for use in radioimmunoassay of small oligopeptides. Methods Enzymol. 1983;103:435–447. doi: 10.1016/s0076-6879(83)03030-x. [DOI] [PubMed] [Google Scholar]
- Youngblood W. W., Lipton M. A., Kizer J. S. TRH-like immunoreactivity in urine, serum and extrahypothalamic brain: non-identity with synthetic pyroglu-hist-pro-NH2 (TRH). Brain Res. 1978 Jul 28;151(1):99–116. doi: 10.1016/0006-8993(78)90953-8. [DOI] [PubMed] [Google Scholar]
- Youngblood W. W., Moray L. J., Busby W. H., Kizer J. S. Bovine serum albumin-GABA-His-Pro-NH2: an immunogen for production of higher affinity antisera for TRH. J Neurosci Methods. 1983 Dec;9(4):367–373. doi: 10.1016/0165-0270(83)90067-5. [DOI] [PubMed] [Google Scholar]


