Abstract
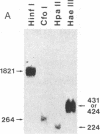
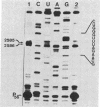
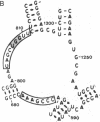
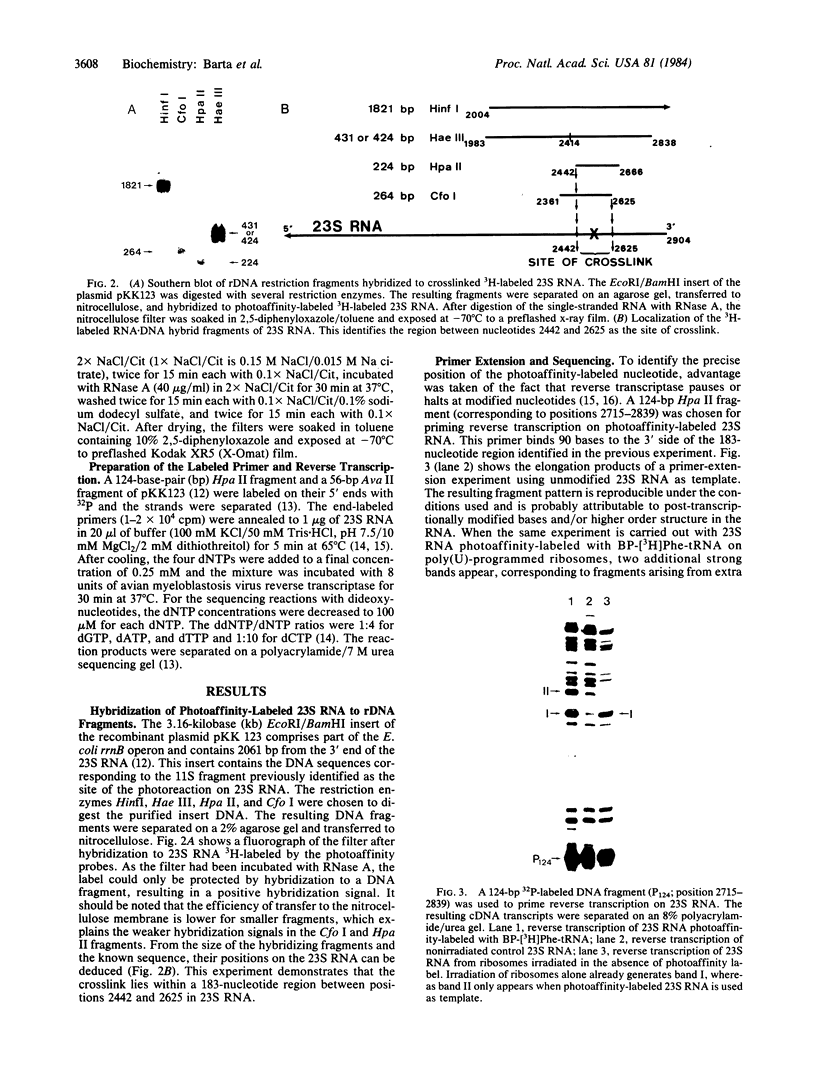
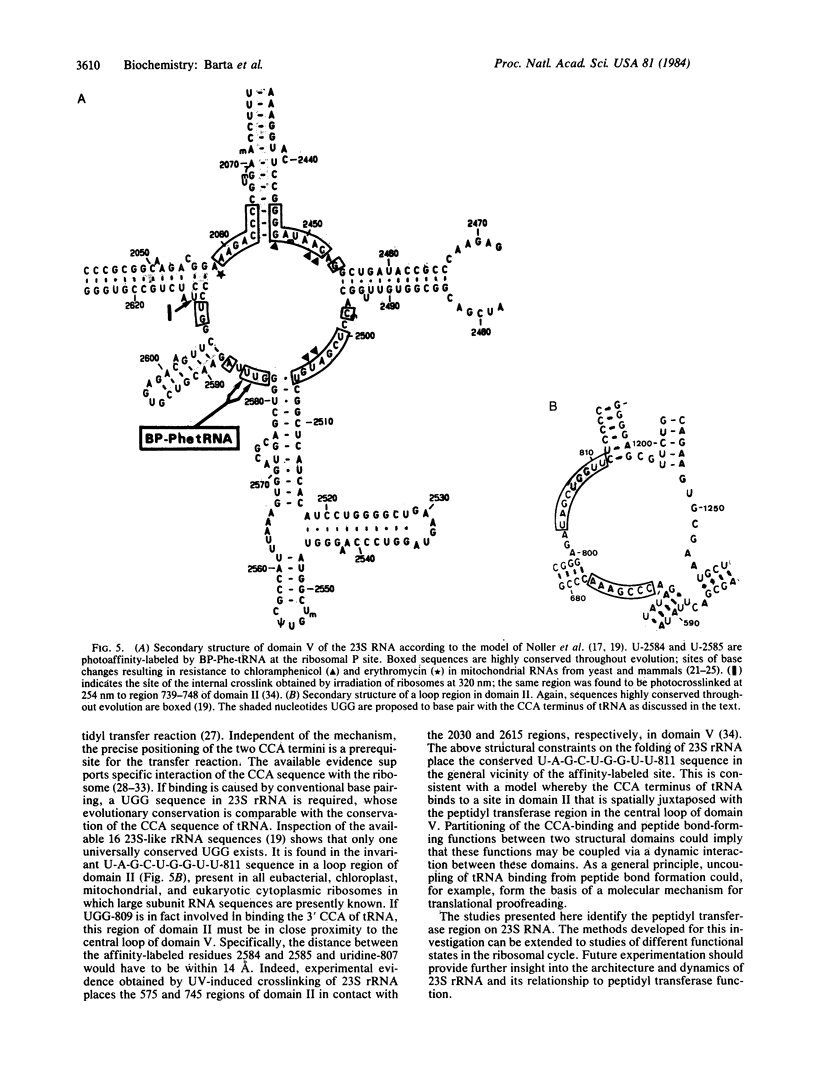
3-(4'-Benzoylphenyl)propionyl[3H] Phe-tRNA bound to the peptidyl site of the ribosome is photo-crosslinked exclusively to 23S RNA on irradiation at 320 nm. The site of reaction has been identified both by hybridization and primer-extension experiments as uridine-2584 and uridine-2585, located within the central loop of domain V according to the secondary structure model of 23S RNA. The fact that the covalently crosslinked tRNA retains its ability to form a peptide bond, together with the proximity of this site to the position of several mutations leading to chloramphenicol or erythromycin resistance strongly argue that this region of the 23S-like rRNAs is an integral component of the peptidyl transferase site. On the basis of these results, and from comparative analysis of the 16 available large subunit rRNA sequences, we propose a model for the functional organization of the peptidyl transferase site involving interaction of domains II and V of 23S rRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barta A., Kuechler E., Branlant C., Sri Widada J., Krol A., Ebel J. P. Photoaffinity labelling of 23 S RNA at the donor-site of the Escherichia coli ribosome. FEBS Lett. 1975 Aug 1;56(1):170–174. doi: 10.1016/0014-5793(75)80134-7. [DOI] [PubMed] [Google Scholar]
- Barta A., Kuechler E. Part of the 23S RNA located in the 11S RNA fragment is a constituent of the ribosomal peptidyltransferase centre. FEBS Lett. 1983 Nov 14;163(2):319–323. doi: 10.1016/0014-5793(83)80844-8. [DOI] [PubMed] [Google Scholar]
- Blanc H., Adams C. W., Wallace D. C. Different nucleotide changes in the large rRNA gene of the mitochondrial DNA confer chloramphenicol resistance on two human cell lines. Nucleic Acids Res. 1981 Nov 11;9(21):5785–5795. doi: 10.1093/nar/9.21.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Wright C. T., Bibb M. J., Wallace D. C., Clayton D. A. Mitochondrial DNA of chloramphenicol-resistant mouse cells contains a single nucleotide change in the region encoding the 3' end of the large ribosomal RNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3789–3793. doi: 10.1073/pnas.78.6.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerná J. Effect of cytidine-5'-monophosphate on peptidyl transferase activity. FEBS Lett. 1975 Oct 15;58(1):94–98. doi: 10.1016/0014-5793(75)80233-x. [DOI] [PubMed] [Google Scholar]
- Douthwaite S., Garrett R. A., Wagner R. Comparison of Escherichia coli tRNAPhe in the free state, in the ternary complex and in the ribosomal A and P sites by chemical probing. Eur J Biochem. 1983 Mar 15;131(2):261–269. doi: 10.1111/j.1432-1033.1983.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Farber N., Cantor C. R. Comparison of the structures of free and ribosome-bound tRNAPhe by using slow tritium exchange. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5135–5139. doi: 10.1073/pnas.77.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Printz M. P. Benzophenone triplet: a new photochemical probe of biological ligand-receptor interactions. Nat New Biol. 1973 Mar 28;242(117):127–128. doi: 10.1038/newbio242127a0. [DOI] [PubMed] [Google Scholar]
- Kearsey S. E., Craig I. W. Altered ribosomal RNA genes in mitochondria from mammalian cells with chloramphenicol resistance. Nature. 1981 Apr 16;290(5807):607–608. doi: 10.1038/290607a0. [DOI] [PubMed] [Google Scholar]
- Kuechler E., Barta A. Aromatic ketone derivatives of aminoacyl-tRNA as photoaffinity labels for ribosomes. Methods Enzymol. 1977;46:676–683. doi: 10.1016/s0076-6879(77)46085-3. [DOI] [PubMed] [Google Scholar]
- Leitner M., Wilchek M., Zamir A. Identification by affinity labeling of potential sites in 23-S rRNA interacting with the 3' end of tRNA. Eur J Biochem. 1982 Jun 15;125(1):49–55. doi: 10.1111/j.1432-1033.1982.tb06649.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Cerná J., Marcker K. A. Ribosome-catalyzed peptidyl transfer: substrate specificity at the P-site. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1042–1049. doi: 10.1073/pnas.61.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus K. H. Structure, assembly, and function of ribosomes. Curr Top Microbiol Immunol. 1982;97:81–155. doi: 10.1007/978-3-642-68318-3_3. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Herr W. Chemical probing of the tRNA--ribosome complex. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2273–2277. doi: 10.1073/pnas.78.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Identification of two erythromycin resistance mutations in the mitochondrial gene coding for the large ribosomal RNA in yeast. Nucleic Acids Res. 1982 Nov 11;10(21):6571–6577. doi: 10.1093/nar/10.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiege W., Glotz C., Brimacombe R. Localisation of a series of intra-RNA cross-links in the secondary and tertiary structure of 23S RNA, induced by ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Nucleic Acids Res. 1983 Mar 25;11(6):1687–1706. doi: 10.1093/nar/11.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundari R. M., Pelka H., Schulman L. H. Structural requirements of Escherichia coli formylmethionyl transfer ribonucleic acid for ribosome binding and initiation of protein synthesis. J Biol Chem. 1977 Jun 10;252(11):3941–3944. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. A sequence from Drosophila melanogaster 18S rRNA bearing the conserved hypermodified nucleoside am psi: analysis by reverse transcription and high-performance liquid chromatography. Nucleic Acids Res. 1981 Apr 10;9(7):1723–1741. doi: 10.1093/nar/9.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Reverse transcriptase pauses at N2-methylguanine during in vitro transcription of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3751–3754. doi: 10.1073/pnas.76.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]