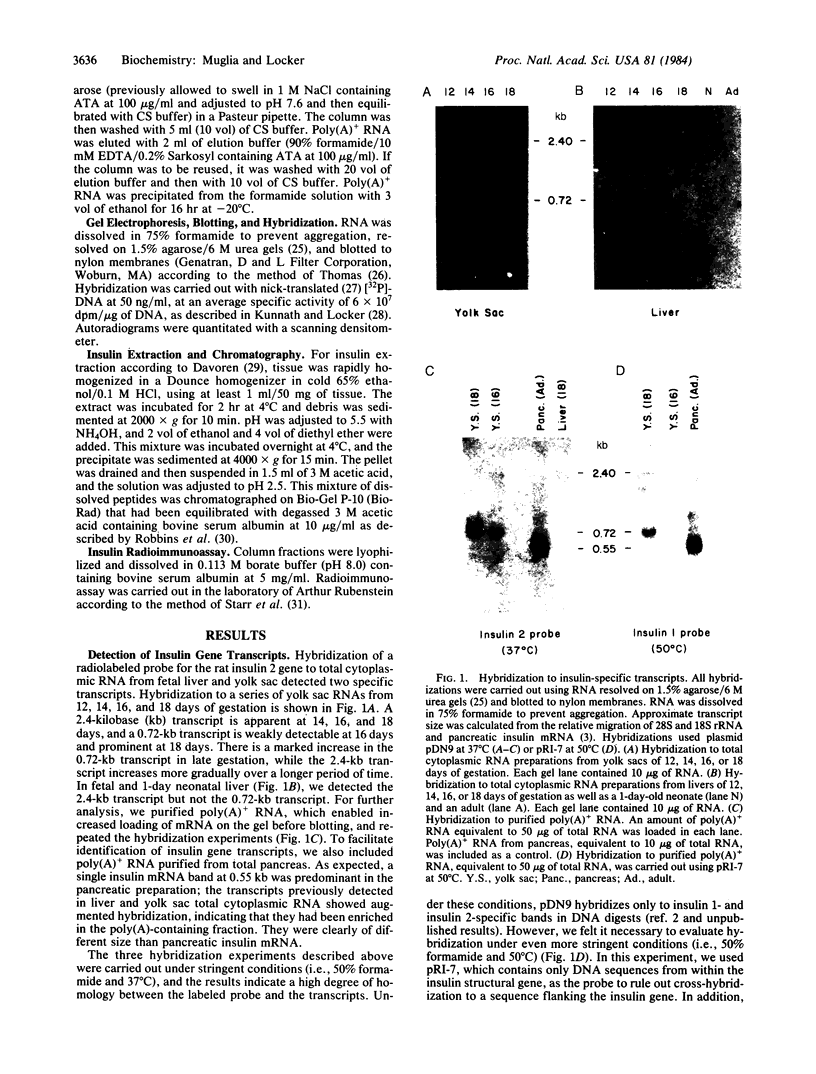
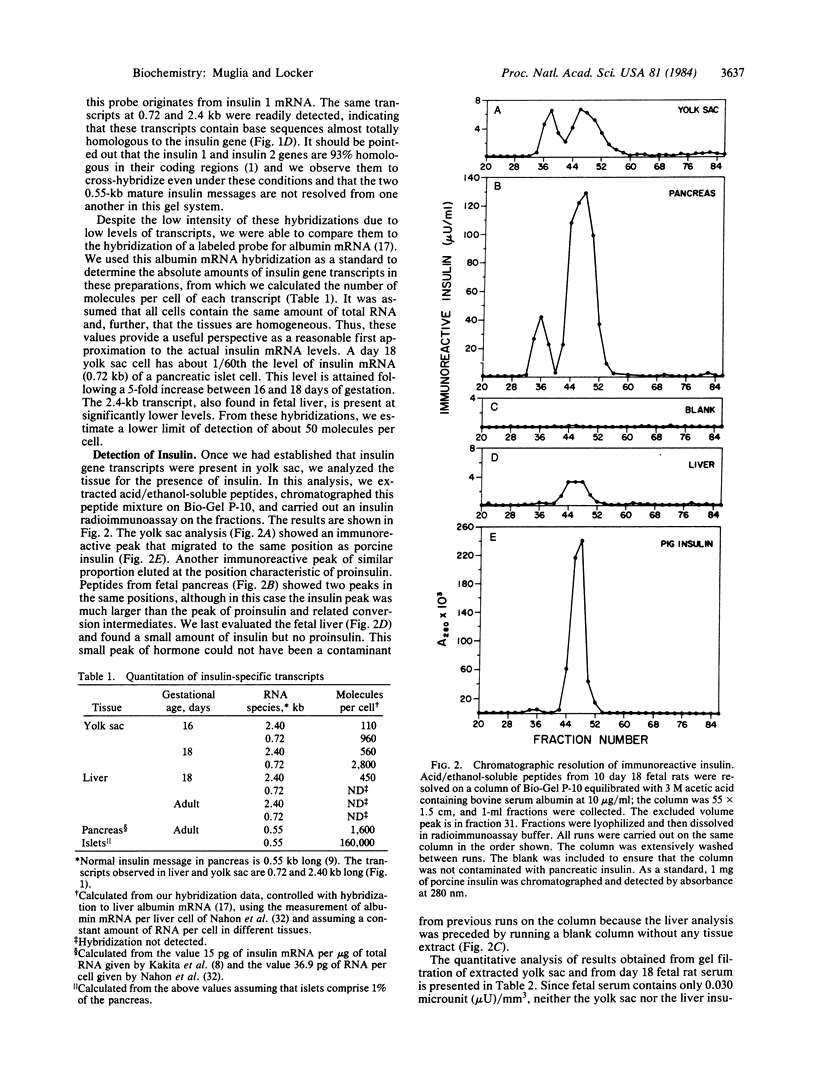
Abstract
Analysis of gene expression in rat yolk sac, a primitive endodermal structure, revealed a low level of developmentally regulated insulin production. At 14 days of gestation, a 2.4-kilobase (kb) RNA species hybridized to cloned insulin gene probes. This species increased throughout gestation. At 16 days, a second transcript of 0.72 kb became visible and, by 18 days, the 0.72-kb transcript predominated. In the pancreas, the fully processed insulin mRNA is 0.55 kb long. Over the same time period in the fetal liver (also a tissue of endodermal origin, as is the pancreas), only the 2.4-kb transcript was detected; no hybridizing transcripts were detected in adult liver RNA. Gel filtration studies and insulin radioimmunoassay of acid/ethanol-soluble peptides showed approximately equal amounts of proinsulin and insulin in 18-day yolk sac, a result suggesting that the transcripts in this tissue are translated. On the other hand, a lower level of insulin and the lack of proinsulin in fetal liver were compatible with a pancreatic origin of hepatocyte insulin by receptor binding rather than intrahepatic insulin synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Kwok S. C., Steiner D. F. The biosynthesis of insulin: some genetic and evolutionary aspects. Diabetes Care. 1981 Jan-Feb;4(1):4–10. doi: 10.2337/diacare.4.1.4. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Diamond D., Smith S., Pünter J., Schöne H. H., Goodman H. M. Disproportionate expression of the two nonallelic rat insulin genes in a pancreatic tumor is due to translational control. Cell. 1982 Dec;31(3 Pt 2):531–542. doi: 10.1016/0092-8674(82)90309-9. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Applewhite G. T., Underwood L. E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980 Mar 15;75(2):315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Eng J., Yalow R. S. Evidence against extrapancreatic insulin synthesis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4576–4578. doi: 10.1073/pnas.78.7.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings S. J., Rotwein P., Chirgwin J. M., Scharp D., Permutt M. A. Analysis of insulin gene expression in human pancreas. Diabetes. 1983 Aug;32(8):777–780. doi: 10.2337/diab.32.8.777. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrankova J., Schmechel D., Roth J., Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Kakita K., Giddings S. J., Rotwein P. S., Permutt M. A. Insulin gene expression in the developing rat pancreas. Diabetes. 1983 Aug;32(8):691–696. doi: 10.2337/diab.32.8.691. [DOI] [PubMed] [Google Scholar]
- Kunnath L., Locker J. Variable methylation of the ribosomal RNA genes of the rat. Nucleic Acids Res. 1982 Jul 10;10(13):3877–3892. doi: 10.1093/nar/10.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub O., Rall L., Bell G. I., Rutter W. J. Expression of the human insulin gene in an alternate mammalian cell and in cell extracts. J Biol Chem. 1983 May 25;258(10):6037–6042. [PubMed] [Google Scholar]
- Laub O., Rutter W. J. Expression of the human insulin gene and cDNA in a heterologous mammalian system. J Biol Chem. 1983 May 25;258(10):6043–6050. [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Nahon J. L., Gal A., Frain M., Sell S., Sala-Trepat J. M. No evidence for post-transcriptional control of albumin and alpha-fetoprotein gene expression in developing rat liver neoplasia. Nucleic Acids Res. 1982 Mar 25;10(6):1895–1911. doi: 10.1093/nar/10.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D. A., Chou J., MacKrell A. J., Casadaban M. J., Steiner D. F. Expression of a preproinsulin-beta-galactosidase gene fusion in mammalian cells. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5198–5202. doi: 10.1073/pnas.80.17.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes B. E., Mevarech M., Stein R., Agarwal K. L. Detection and partial sequence analysis of gastrin mRNA by using an oligodeoxynucleotide probe. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1770–1774. doi: 10.1073/pnas.76.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- Robbins D. C., Blix P. M., Rubenstein A. H., Kanazawa Y., Kosaka K., Tager H. S. A human proinsulin variant at arginine 65. Nature. 1981 Jun 25;291(5817):679–681. doi: 10.1038/291679a0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig J. L., Havrankova J., Lesniak M. A., Brownstein M., Roth J. Insulin is ubiquitous in extrapancreatic tissues of rats and humans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):572–576. doi: 10.1073/pnas.77.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F. The Banting Memorial Lecture 1976. Insulin today. Diabetes. 1977 Apr;26(4):322–340. doi: 10.2337/diab.26.4.322. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]