Abstract
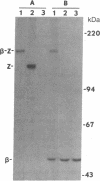
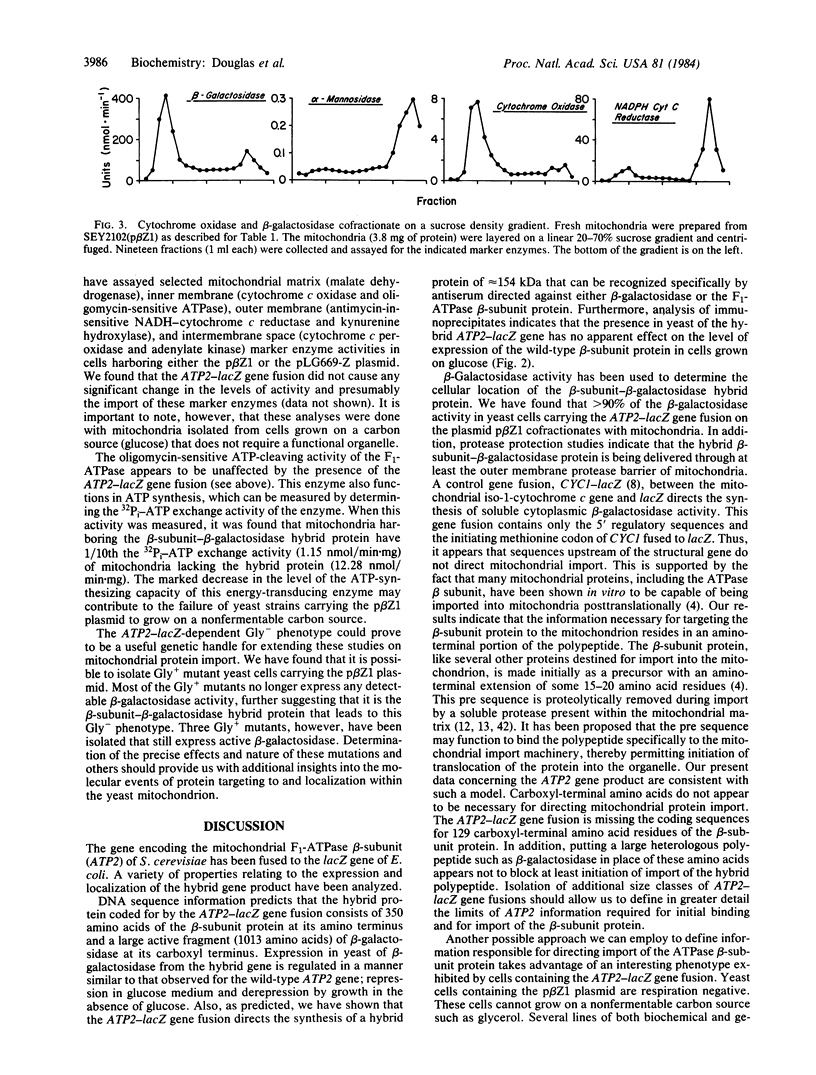
The gene coding for the yeast mitochondrial F1-ATPase beta subunit (ATP2) has been fused to the Escherichia coli lacZ gene. The chimeric ATP2-lacZ gene codes for a hybrid protein consisting of some 350 amino acids of the F1-ATPase beta subunit at its amino terminus and a large enzymatically active portion of the lacZ gene product, beta-galactosidase (beta-D-galactoside galactohydrolase, EC 3.2.1.23), at its carboxyl terminus. The beta-subunit-beta-galactosidase hybrid protein is expressed in both E. coli and yeast. In yeast, this hybrid molecule is targeted to the mitochondrion and is protected in isolated mitochondria from added protease under conditions in which an outer membrane enzymatic marker is digested. Yeast cells carrying the ATP2-lacZ gene fusion on plasmid p beta Z1 are unable to grow on a nonfermentable carbon source. Upon loss of the p beta Z1 plasmid, growth of the cured host strain on the nonfermentable substrate is restored. In the presence of the beta-subunit-beta-galactosidase hybrid protein, the energy-transducing capacity of the mitochondrial membrane as measured by the 32Pi-ATP exchange reaction is only 9% of that measured in the absence of the gene fusion product. The results indicate that it is the presence of the beta-subunit-beta-galactosidase hybrid protein within mitochondria that interferes with function(s) essential for respiratory growth. These observations open up the prospect of genetic characterization of the signals and cellular machinery responsible for mitochondrial protein delivery.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandlow W. Membrane separation and biogenesis of the outer membrane of yeast mitochondria. Biochim Biophys Acta. 1972 Sep 1;282(1):105–122. doi: 10.1016/0005-2736(72)90315-x. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Silhavy T. J. Information within the mature LamB protein necessary for localization to the outer membrane of E coli K12. Cell. 1983 Apr;32(4):1325–1335. doi: 10.1016/0092-8674(83)90313-6. [DOI] [PubMed] [Google Scholar]
- Böhni P. C., Daum G., Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem. 1983 Apr 25;258(8):4937–4943. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Djavadi-Ohaniance L., Rudin Y., Schatz G. Identification of enzymically inactive apocytochrome c peroxidase in anaerobically grown Saccharomyces cerevisiae. J Biol Chem. 1978 Jun 25;253(12):4402–4407. [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Koh Y., Dockter M. E., Schatz G. Aurovertin binds to the beta subunit of yeast mitochondrial ATPase. J Biol Chem. 1977 Dec 10;252(23):8333–8335. [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Schekman R., Flessel M. C., Thorner J. An MF alpha 1-SUC2 (alpha-factor-invertase) gene fusion for study of protein localization and gene expression in yeast. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7080–7084. doi: 10.1073/pnas.80.23.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Molecular components of the signal sequence that function in the initiation of protein export. J Cell Biol. 1982 Dec;95(3):689–696. doi: 10.1083/jcb.95.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAda P. C., Douglas M. G. A neutral metallo endoprotease involved in the processing of an F1-ATPase subunit precursor in mitochondria. J Biol Chem. 1982 Mar 25;257(6):3177–3182. [PubMed] [Google Scholar]
- McAda P. C., Douglas M. G. A yeast mitochondrial chelator-sensitive protease that processes cytoplasmically synthesized protein precursors: isolation from yeast and assay. Methods Enzymol. 1983;97:337–344. doi: 10.1016/0076-6879(83)97146-x. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J Biol Chem. 1982 Nov 10;257(21):13056–13061. [PubMed] [Google Scholar]
- Rose M., Casadaban M. J., Botstein D. Yeast genes fused to beta-galactosidase in Escherichia coli can be expressed normally in yeast. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2460–2464. doi: 10.1073/pnas.78.4.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ G., KLIMA J. TRIPHOSPHOPYRIDINE NUCLEOTIDE: CYTOCHROME C REDUCTASE OF SACCHAROMYCES CEREVISIAE: A "MICROSOMAL" ENZYME. Biochim Biophys Acta. 1964 Mar 9;81:448–461. doi: 10.1016/0926-6569(64)90130-0. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Muller J., Kunapuli S. P., Douglas M. G. Nuclear genes coding the yeast mitochondrial adenosine triphosphatase complex. Isolation of ATP2 coding the F1-ATPase beta subunit. J Biol Chem. 1983 Oct 10;258(19):11465–11470. [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely E., Montgomery D. L. Glucose represses transcription of Saccharomyces cerevisiae nuclear genes that encode mitochondrial components. Mol Cell Biol. 1984 May;4(5):939–946. doi: 10.1128/mcb.4.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. D., Griesenbeck T. A., Douglas M. G. The yeast mitochondrial adenosine triphosphatase complex. Subunit stoichiometry and physical characterization. J Biol Chem. 1980 Jun 10;255(11):5461–5467. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Sierra M. F. Assembly of the mitochondrial membrane system. VII. Synthesis and integration of F 1 subunits into the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6511–6516. [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem. 1969 Sep 25;244(18):5027–5033. [PubMed] [Google Scholar]