Abstract
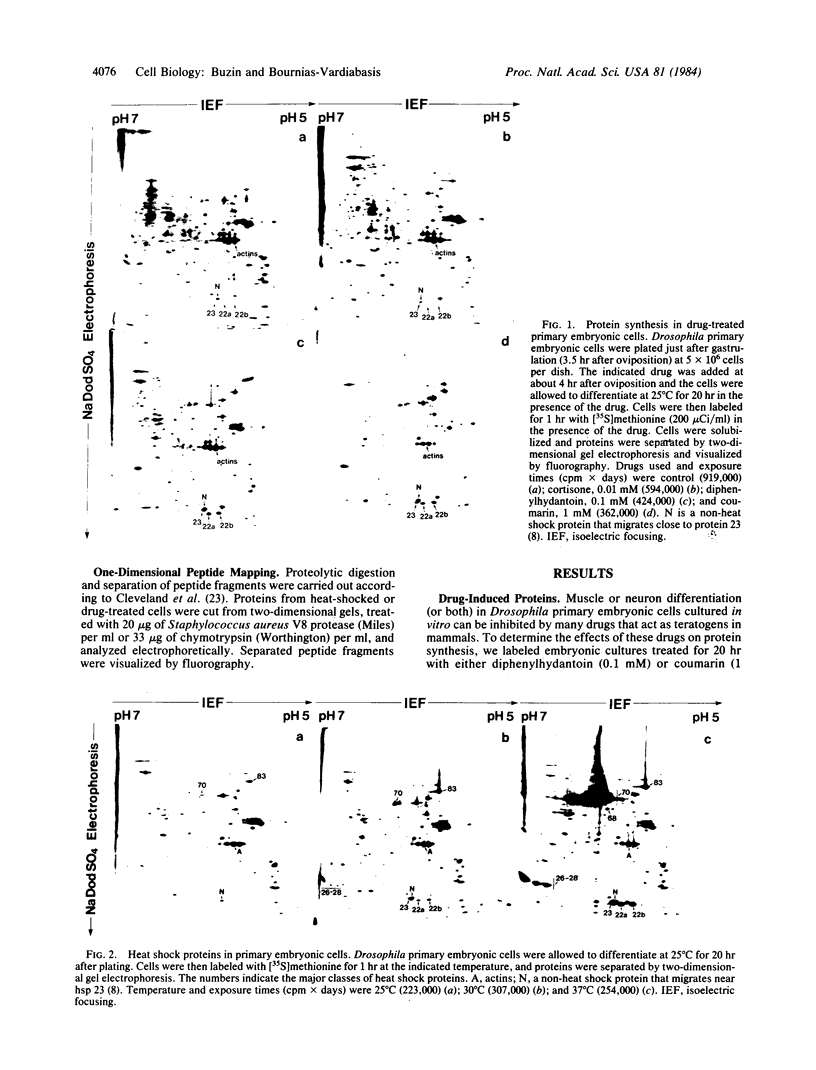
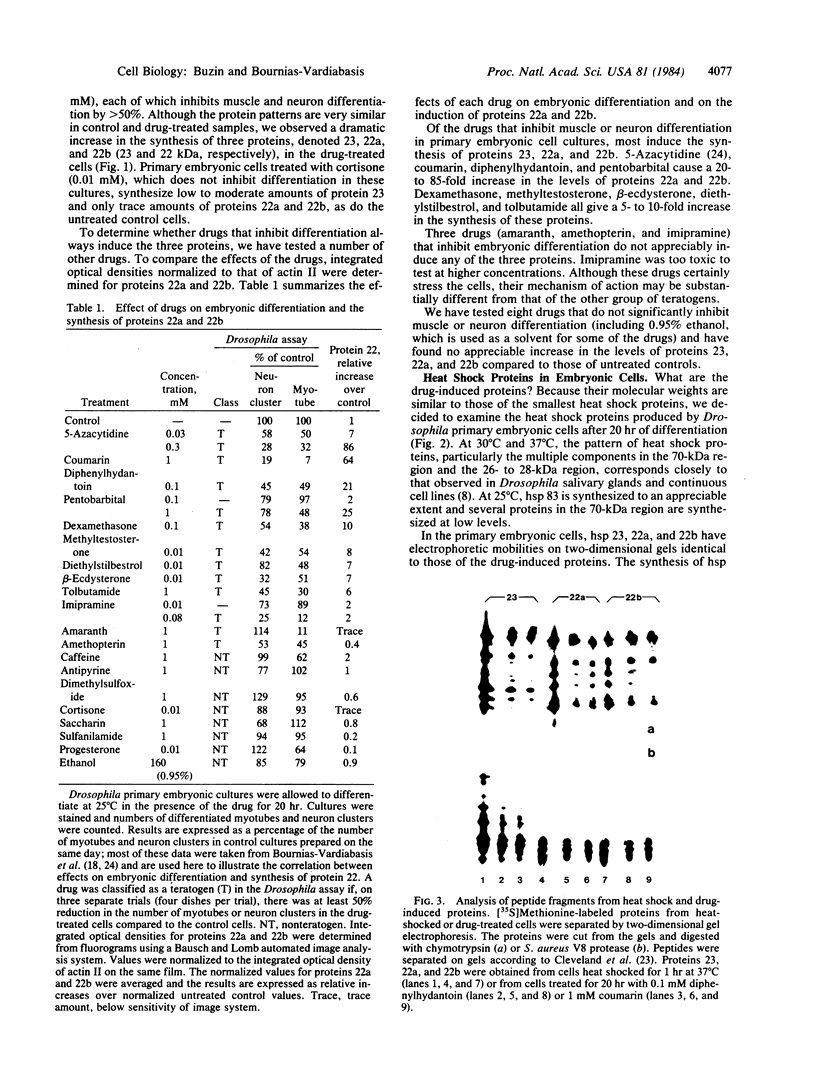
Drosophila embryonic cells placed into culture just after gastrulation differentiate in vitro over the next 24 hr. A number of drugs that are teratogenic in mammalian systems have been found to inhibit muscle or neuron differentiation (or both) in these developing cultures. We have examined, by two-dimensional gel electrophoresis, the effects of these drugs on protein synthesis in embryonic cells. For nine teratogens tested, cells treated for 20 hr with the drug show a dramatic induction of three proteins of about 20 kilodaltons, in addition to the normal proteins synthesized by untreated cells. Three teratogens as well as all eight nonteratogens tested did not show this induction. The induced proteins appear to be identical to three of the heat shock proteins (hsp 23, 22a, and 22b), as shown by electrophoretic mobilities and peptide mapping by partial proteolysis. A 37 degrees C heat shock of the embryonic cells produces the full complement of heat shock proteins, whereas drug-treated cells induce only the subset hsp 23, 22a, and 22b but not hsp 26 or 27. beta-Ecdysterone, the Drosophila molting hormone, also inhibits embryonic differentiation and induces hsp 23, 22a, and 22b, a partial subset of the heat shock proteins (hsp 22, 23, 26, and 27) induced by the hormone in imaginal discs and some Drosophila continuous cell lines. Dose-response studies of several drugs show a correlation between the degree of inhibition of differentiation and the level of induction of hsp 23, 22a, and 22b. The induction of heat shock proteins by drugs may reflect specific types of stress that can also give rise to teratogenesis.
Full text
PDF


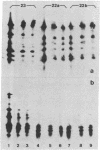
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bournias-Vardiabasis N., Teplitz R. L., Chernoff G. F., Seecof R. L. Detection of teratogens in the Drosophila embryonic cell culture test: assay of 100 chemicals. Teratology. 1983 Aug;28(1):109–122. doi: 10.1002/tera.1420280114. [DOI] [PubMed] [Google Scholar]
- Bournias-Vardiabasis N., Teplitz R. L. Use of Drosophila embryo cell cultures as an in vitro teratogen assay. Teratog Carcinog Mutagen. 1982;2(3-4):333–341. doi: 10.1002/1520-6866(1990)2:3/4<333::aid-tcm1770020315>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Cheney C. M., Shearn A. Developmental regulation of Drosophila imaginal disc proteins: synthesis of a heat shock protein under non-heat-shock conditions. Dev Biol. 1983 Feb;95(2):325–330. doi: 10.1016/0012-1606(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J. Four Drosophila heat shock genes at 67B: characterization of recombinant plasmids. Nucleic Acids Res. 1980 Oct 10;8(19):4441–4457. doi: 10.1093/nar/8.19.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. C., Berger E. M. Synthesis of low molecular weight heat shock peptides stimulated by ecdysterone in a cultured Drosophila cell line. Proc Natl Acad Sci U S A. 1982 Feb;79(3):855–859. doi: 10.1073/pnas.79.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. C., Berger E., Sirotkin K., Yund M. A., Osterbur D., Fristrom J. Ecdysterone induces the transcription of four heat-shock genes in Drosophila S3 cells and imaginal discs. Dev Biol. 1982 Oct;93(2):498–507. doi: 10.1016/0012-1606(82)90137-3. [DOI] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Seecof R. L., Alléaume N., Teplitz R. L., Gerson I. Differentiation of neurons and myocytes in cell cultures made from Drosophila gastrulae. Exp Cell Res. 1971 Nov;69(1):161–173. doi: 10.1016/0014-4827(71)90321-1. [DOI] [PubMed] [Google Scholar]
- Seecof R. L., Donady J. J., Teplitz R. L. Differentiation of Drosophila neuroblasts to form ganglion-like clusters of neurons in vitro. Cell Differ. 1973 Jul;2(3):143–149. doi: 10.1016/0045-6039(73)90014-6. [DOI] [PubMed] [Google Scholar]
- Seecof R. L., Gerson I., Donady J. J., Teplitz R. L. Drosophilia myogenesis in vitro: the genesis of "small" myocytes and myotubes. Dev Biol. 1973 Dec;35(2):250–261. doi: 10.1016/0012-1606(73)90022-5. [DOI] [PubMed] [Google Scholar]
- Seecof R. L., Teplitz R. L., Gerson I., Ikeda K., Donady J. Differentiation of neuromuscular junctions in cultures of embryonic Drosophila cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):566–570. doi: 10.1073/pnas.69.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Davidson N. Developmentally regulated transcription from Drosophila melanogaster chromosomal site 67B. Dev Biol. 1982 Jan;89(1):196–210. doi: 10.1016/0012-1606(82)90307-4. [DOI] [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975 Apr;4(4):395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Goldschmidt-Clermont M., Southgate R., Tissières A., Levis R., Gehring W. A DNA segment isolated from chromosomal site 67B in D. melanogaster contains four closely linked heat-shock genes. Cell. 1981 Jan;23(1):261–270. doi: 10.1016/0092-8674(81)90290-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]