Abstract
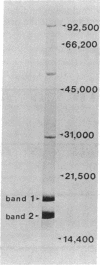
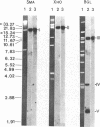
Antibodies raised against mixtures of phycobilisome polypeptides from the eukaryotic alga Cyanidium caldarium were used in an immunological screen to detect expression of phycobiliprotein genes in an Escherichia coli library containing segments of plastid (chloroplast, cyanelle) DNA from another eukaryotic alga, Cyanophora paradoxa. The four candidate clones obtained were mapped by restriction analysis and found to be overlapping. The clone with the smallest insert (1.4 kilobases) was partially sequenced and a coding region similar to the carboxyl terminus of the phycobiliprotein subunit β-phycocyanin was found. The coding region for the β-phycocyanin gene in C. paradoxa has been mapped to the small single copy region on the cyanelle genome, and its orientation has been determined. A short probe unique to a conserved chromophore binding site shared by at least two phycobiliprotein subunits has now been generated from the carboxyl terminus of the β-phycocyanin gene. This probe may be useful in identifying specific phycobiliprotein subunit genes, β-phycocyanin, β-phycoerythrocyanin, and possibly β-phycoerythrin, in other eukaryotic algae and in prokaryotic cyanobacteria.
Keywords: phycobilisome, β-phycocyanin, immunological screening, chloroplast genes
Full text
PDF


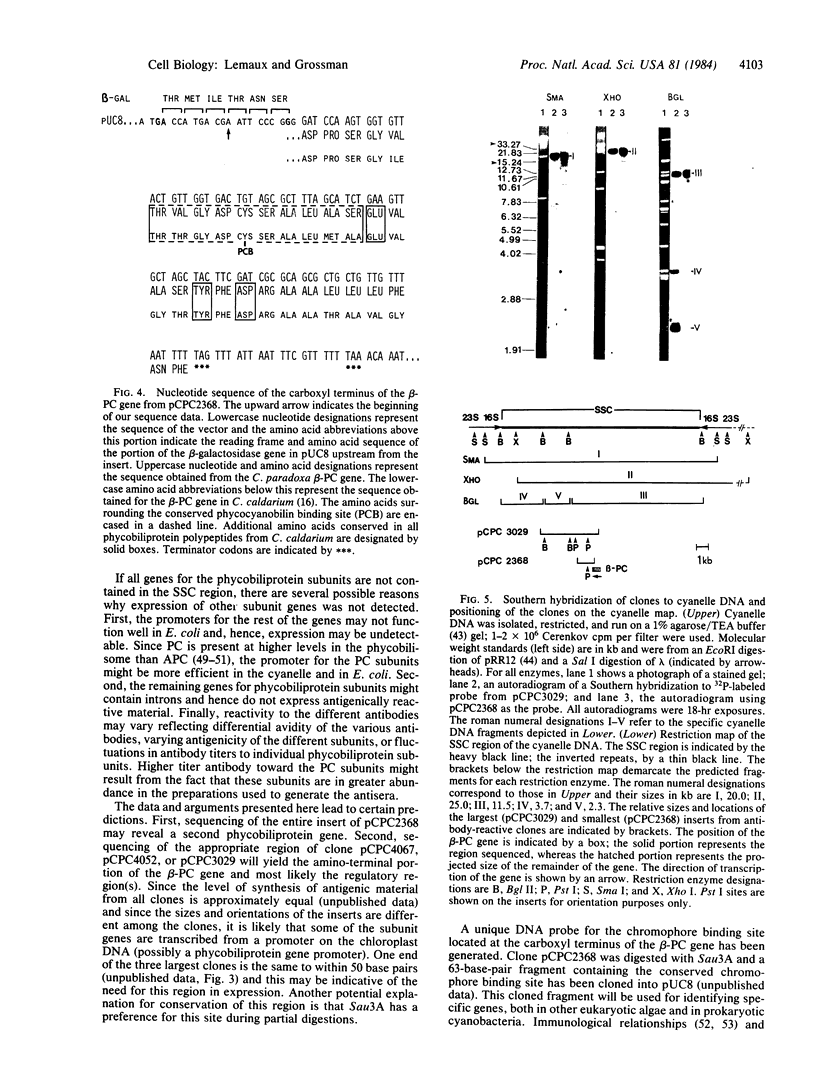

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belford H. S., Offner G. D., Troxler R. F. Phycobiliprotein synthesis in the unicellular rhodophyte, Cyanidium caldarium. Cell-free translation of the mRNAs for the alpha and beta subunit polypeptides of phycocyanin. J Biol Chem. 1983 Apr 10;258(7):4503–4510. [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., Glazer A. N., Eiserling F. A. Characterization and structural properties of the major biliproteins of Anabaena sp. Arch Microbiol. 1976 Oct 11;110(1):61–75. doi: 10.1007/BF00416970. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., Hixson C. S., Glazer A. N. Structural studies on phycobiliproteins III. Comparison of bilin-containing peptides from the beta subunits of C-phycocyanin, R-phycocyanin, and phycoerythrocyanin. J Biol Chem. 1978 Jan 10;253(1):220–225. [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Selection of specific clones from colony banks by suppression or complementation tests. Methods Enzymol. 1979;68:396–408. doi: 10.1016/0076-6879(79)68029-1. [DOI] [PubMed] [Google Scholar]
- Craig I. W., Carr N. G. C-phycocyanin and allophycocyanin in two species of blue-green algae. Biochem J. 1968 Jan;106(2):361–366. doi: 10.1042/bj1060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Glazer A. N. The amino acid sequence of the beta subunit of allophycocyanin. J Biol Chem. 1981 Sep 25;256(18):9558–9566. [PubMed] [Google Scholar]
- Egelhoff T., Grossman A. Cytoplasmic and chloroplast synthesis of phycobilisome polypeptides. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3339–3343. doi: 10.1073/pnas.80.11.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H. A., Cohen S. N., McDevitt H. O. A sensitive radioimmunoassay for detecting products translated from cloned DNA fragments. Cell. 1978 Apr;13(4):681–689. doi: 10.1016/0092-8674(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Frank G., Sidler W., Widmer H., Zuber H. The complete amino acid sequence of both subunits of C-phycocyanin from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1491–1507. doi: 10.1515/bchm2.1978.359.2.1491. [DOI] [PubMed] [Google Scholar]
- Freidenreich P., Apell G. S., Glazer A. N. Structural studies on phycobiliproteins II. C-phycocyanin: amino acid sequence of the beta subunit. Specific cleavage of the alpha subunit. J Biol Chem. 1978 Jan 10;253(1):212–219. [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum: pigment analysis. Biochemistry. 1974 Jul 2;13(14):2960–2966. doi: 10.1021/bi00711a027. [DOI] [PubMed] [Google Scholar]
- Gatenby A. A., Castleton J. A., Saul M. W. Expression in E. coli of maize and wheat chloroplast genes for large subunit of ribulose bisphosphate carboxylase. Nature. 1981 May 14;291(5811):117–121. doi: 10.1038/291117a0. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Bryant D. A. Allophycocyanin B (lambdamax 671, 618 nm): a new cyanobacterial phycobiliprotein. Arch Microbiol. 1975 Jun 20;104(1):15–22. doi: 10.1007/BF00447294. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G., Stanier R. Y. Characterization of phycoerythrin from a Cryptomonas sp. Arch Mikrobiol. 1971;80(1):1–18. doi: 10.1007/BF00410574. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Cohen-Bazire G., Stanier R. Y. Comparative immunology of algal biliproteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3005–3008. doi: 10.1073/pnas.68.12.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W., Schneider H. Isolation and characterization of disc-shaped phycobilisomes from the red alga Rhodella violacea. Arch Microbiol. 1977 Feb 4;112(1):61–67. doi: 10.1007/BF00446655. [DOI] [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Structure of the 18 S core-rod subassembly of the Synechococcus 6301 phycobilisome. J Biol Chem. 1983 Jan 25;258(2):894–901. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke H., Löffelhardt W., Bohnert H. J. Partial characterization of the genome of the 'endosymbiotic' cyanelles from Cyanophora paradoxa. FEBS Lett. 1980 Mar 10;111(2):347–352. doi: 10.1016/0014-5793(80)80824-6. [DOI] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F. Primary structure of allophycocyanin from the unicellular rhodophyte, Cyanidium caldarium. The complete amino acid sequences of the alpha and beta subunits. J Biol Chem. 1983 Aug 25;258(16):9931–9940. [PubMed] [Google Scholar]
- Redlinger T., Gantt E. A M(r) 95,000 polypeptide in Porphyridium cruentum phycobilisomes and thylakoids: Possible function in linkage of phycobilisomes to thylakoids and in energy transfer. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5542–5546. doi: 10.1073/pnas.79.18.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanak N., Cramer J. H., Rownd R. H. EcoRI restriction endonuclease map of the composite R plasmid NR1. J Bacteriol. 1976 Jul;127(1):619–636. doi: 10.1128/jb.127.1.619-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Ehrhardt M. M., Brown-Mason A. S., Offner G. D. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. II. Complete amino acid sequence of the beta subunit. J Biol Chem. 1981 Dec 10;256(23):12176–12184. [PubMed] [Google Scholar]
- Troxler R. F., Foster J. A., Brown A. S., Franzblau C. The alpha and beta subunits of Cyanidium caldarium phycocyanin: properties and amino acid sequences at the amino terminus. Biochemistry. 1975 Jan 28;14(2):268–274. doi: 10.1021/bi00673a012. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Whittle D. J., Kilburn D. G., Warren R. A., Miller R. C., Jr Molecular cloning of a Cellulomonas fimi cellulose gene in Escherichia coli. Gene. 1982 Feb;17(2):139–145. doi: 10.1016/0378-1119(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gingrich J. C., Glazer A. N. Cyanobacterial phycobilisomes. Particles from Synechocystis 6701 and two pigment mutants. J Cell Biol. 1980 Jun;85(3):558–566. doi: 10.1083/jcb.85.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V. P., Glazer A. N. Structural studies on phycobiliproteins. I. Bilin-containing peptides of C-phycocyanin. J Biol Chem. 1978 Jan 10;253(1):202–211. [PubMed] [Google Scholar]