Abstract
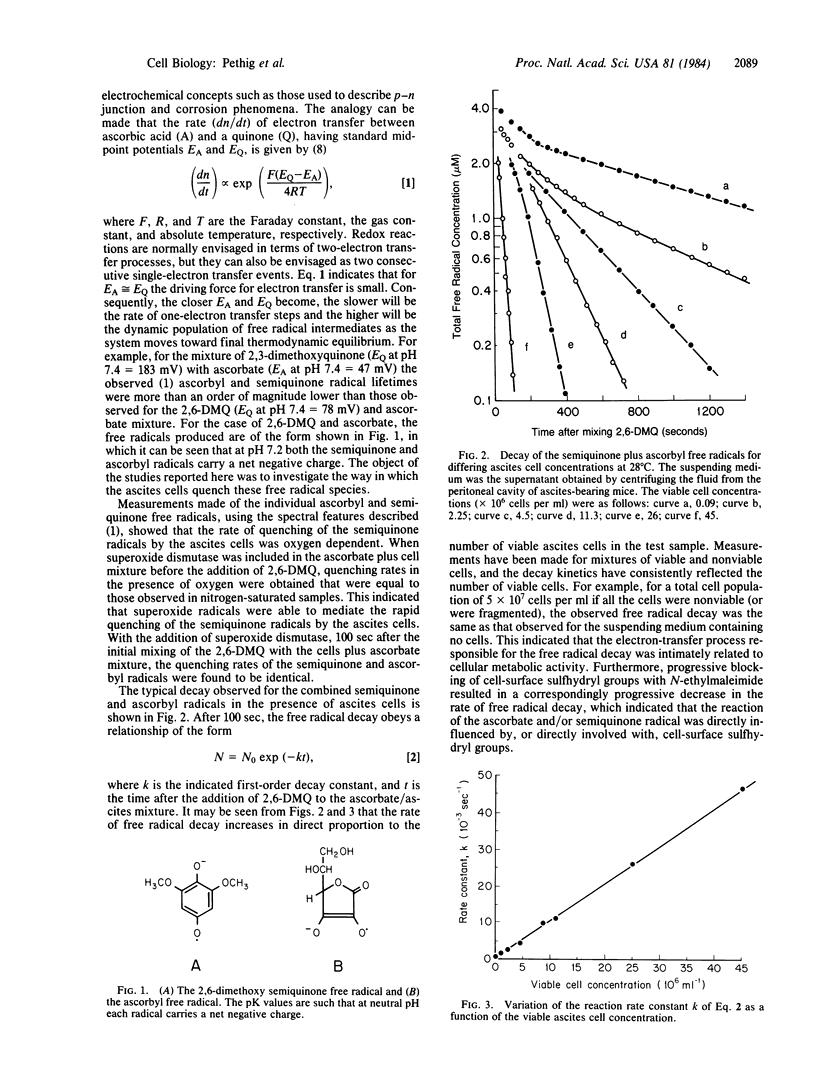
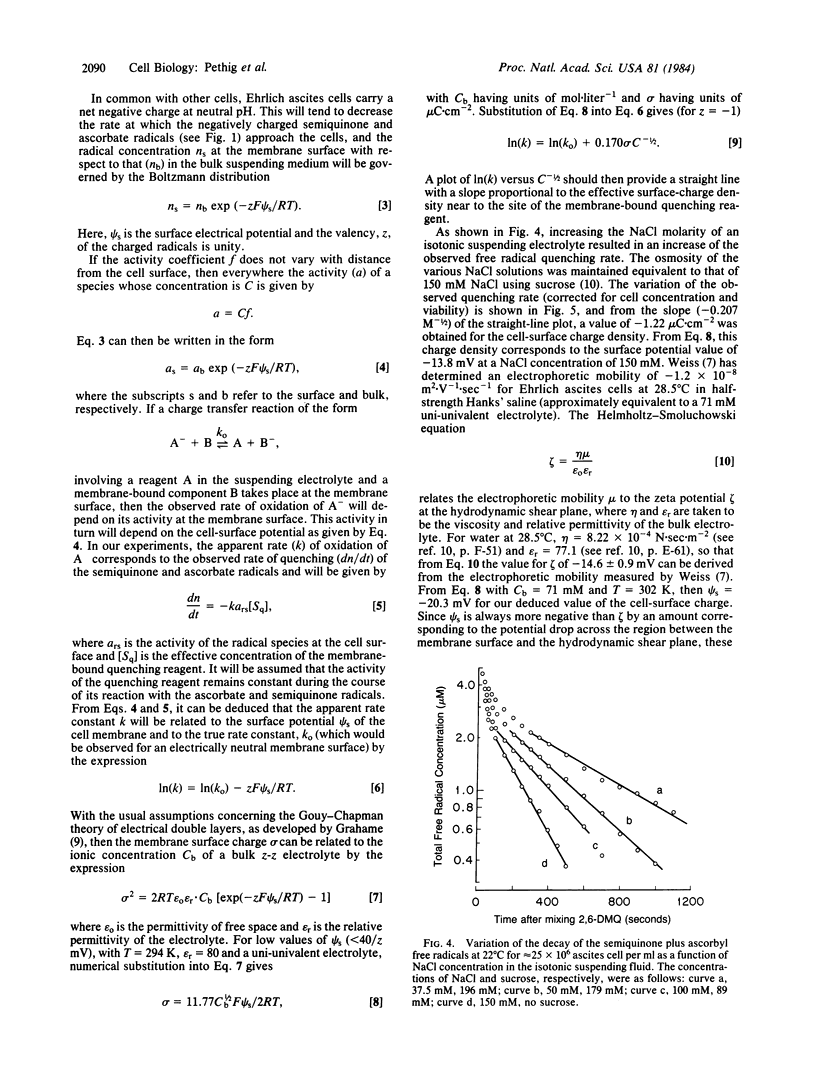
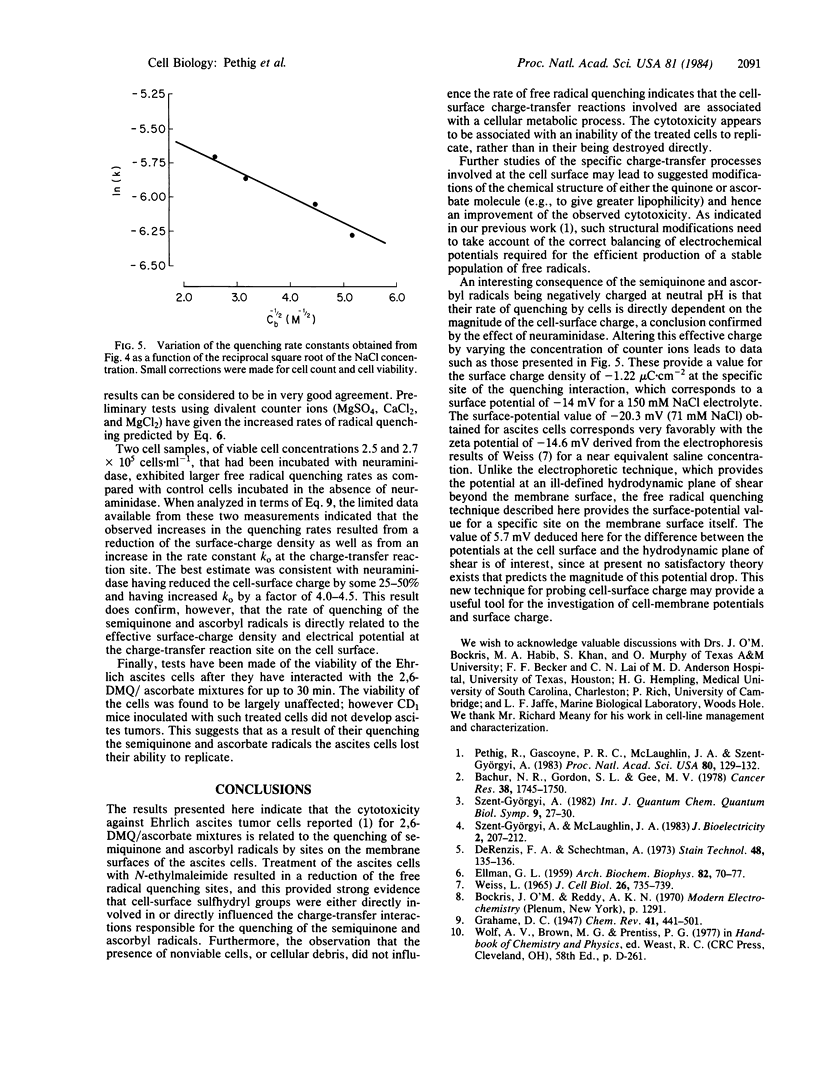
The rate of quenching by Ehrlich ascites cells of anionic 2,6-dimethoxy-p-semiquinone and ascorbyl free radicals is investigated as a function of cell concentration, the blocking of cell-surface sulfhydryl groups by N-ethylmaleimide, and the reduction of cell-surface charge by neuraminidase. The rate of quenching is found to be proportional to cell viability and to the number of free cell-surface sulfhydryl groups. The enzymatic action of neuraminidase results in an increase of the free radical quenching rate, indicating that this rate can be used as a probe of cell-surface charge. Measurements as a function of the ionic strength of the suspending electrolyte gave a value of -1.22 microC X cm-2 for the charge density at the ascites cell surface. This is equivalent to a surface membrane potential of -14 mV for a 150 mM NaCl electrolyte and is a value in good agreement with published electrophoresis data.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- DeRenzis F. A., Schechtman A. Staining by neutral red and trypan blue in sequence for assaying vital and nonvital cultured cells. Stain Technol. 1973 May;48(3):135–136. doi: 10.3109/10520297309116602. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Pethig R., Gascoyne P. R., McLaughlin J. A., Szent-Györgyi A. Ascorbate-quinone interactions: electrochemical, free radical, and cytotoxic properties. Proc Natl Acad Sci U S A. 1983 Jan;80(1):129–132. doi: 10.1073/pnas.80.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]


