Summary
Type I interferons (IFNs) are considered to be the universal mechanism by which viral infections are controlled. However, many IFN-stimulated genes (ISGs) rely on antiviral pathways that are toxic to host cells, which may be detrimental in non-renewable cell types, such as neurons. We show that dorsal root ganglionic (DRG) neurons produce little type I IFNs in response to infection with a neurotropic virus, herpes simplex type 1 (HSV-1). Further, type I IFN treatment fails to completely block HSV-1 replication and to induce IFN-primed cell death in neurons. We find that DRG neurons require autophagy to limit HSV-1 replication both in vivo and in vitro. In contrast, mucosal epithelial cells and other mitotic cells respond robustly to type I IFNs and do not require autophagy to control viral replication. These findings reveal a fundamental difference in the innate antiviral strategies employed by neurons and mitotic cells to control HSV-1 infection.
Introduction
Type I interferon (IFN) plays a key role in innate antiviral defense through the up-regulation of numerous ISGs that antagonize viral replication and activate adaptive immunity (Stetson and Medzhitov, 2006). Type I IFN receptor signaling activates both antiviral and cell death pathways as key components of host antiviral defense (Sadler and Williams, 2008). In most tissues, the death of virally infected cells is a desired mode of virus containment. Such cell death aids the immune response by preventing further viral replication and spread, aiding cell recruitment, and priming adaptive immune responses (Barber, 2001). However, the vertebrate host cannot tolerate death of neurons as a strategy to control virus infection (Danthi et al., 2010; Griffin, 2010; Khanna et al., 2004; Levine, 2002).
In addition to the interferon response, the autophagy pathway can play an important role in viral containment (Virgin and Levine, 2009). Autophagy is a highly conserved catabolic pathway critical in maintaining basal turnover of cellular components and as a survival mechanism during starvation (Klionsky and Emr, 2000). The autophagy pathway includes several distinct forms of lysosomal degradation including macroautophagy, chaperone-mediated autophagy, and microautophagy. Macroautophagy (hereafter referred to as autophagy) involves the formation of double membrane structures termed autophagosomes that engulf cytoplasmic components including proteins, lipids, and entire organelles (Levine and Klionsky, 2004; Mizushima et al., 2008). Mature autophagosomes efficiently fuse with lysosomes, resulting in rapid, bulk degradation of engulfed material. In addition to its roles in cellular recycling and the starvation response, it is now clear that autophagy is intimately involved in immune responses of eukaryotic organisms (Deretic and Levine, 2009). Thus, autophagy has co-evolved with the vertebrate immune system to enhance immune responses while retaining an evolutionary ancient, innate pathogen degradation capacity.
Notably, most of the in vivo evidence for an antiviral role of autophagy in mammalian hosts is restricted to viruses that specifically target neurons, including Sindbis virus and herpes simplex virus type 1 (HSV-1) (Orvedahl and Levine, 2008). Over-expression of the Atg6 or Beclin-1 protein results in decreased cell death and increased survival of neonatal mice intracranially injected with Sindbis virus (Liang et al., 1998). Moreover, deletion of host Atg genes resulted in a failure to clear Sindbis viral proteins and increased cell death in the absence of autophagy (Orvedahl et al., 2010). HSV-1 encodes ICP34.5, a virulence factor necessary for HSV replication in neurons but not other cell types (Chou et al., 1990; Chou and Roizman, 1992; Whitley et al., 1993). ICP34.5 antagonizes downstream effects of PKR by dephosphorylating the transcription factor e-IF2α, thus enabling viral translation (He et al., 1997). In addition, recent studies demonstrated that ICP34.5 also contains a domain that binds to Beclin-1 (Orvedahl et al., 2007). Deletion of the Beclin-1-binding domain (bbd) of ICP34.5 from HSV-1 effectively abrogated autophagy inhibition by the virus resulting in reduced viral replication, cell death, and mortality following intracranial infection (Orvedahl et al., 2007). In contrast, a limited phenotype of bbd mutant was observed in non-neuronal cell types in vitro (Alexander et al., 2007; Orvedahl et al., 2007). However, the basis for the discrepancy between the striking phenotype seen in vivo versus the lack of effect in cell lines tested in vitro remains unresolved.
During the course of natural genital HSV infection, HSV predominantly infects two cell types: rapidly dividing, short-lived mucosal epithelial cells at the site of infection and the innervating sensory neurons (Fields et al., 2007). While genital herpes has been historically associated with HSV-2, HSV-1 accounts for at least half of new cases in developed countries (Gupta et al., 2007). HSV replication at the primary site of infection can result in uptake of virions by innervating neurons and subsequent entry into the peripheral nervous system (PNS) by retrograde axonal transport, where the virus establishes latency. Here, we utilized a genital infection with HSV-1 to interrogate the innate immune strategies in different cell types. We employ a series of host knockouts and viral mutants to dissect the relative contributions of the type I interferon and autophagy pathways in combating HSV-1 infection in vitro and in vivo. Our findings reveal that autophagy, while dispensable in mitotic cells, plays a fundamental role in antiviral immunity to HSV-1 in neurons.
Results
HSV induces higher levels of type I IFN in mitotic cells than in neurons
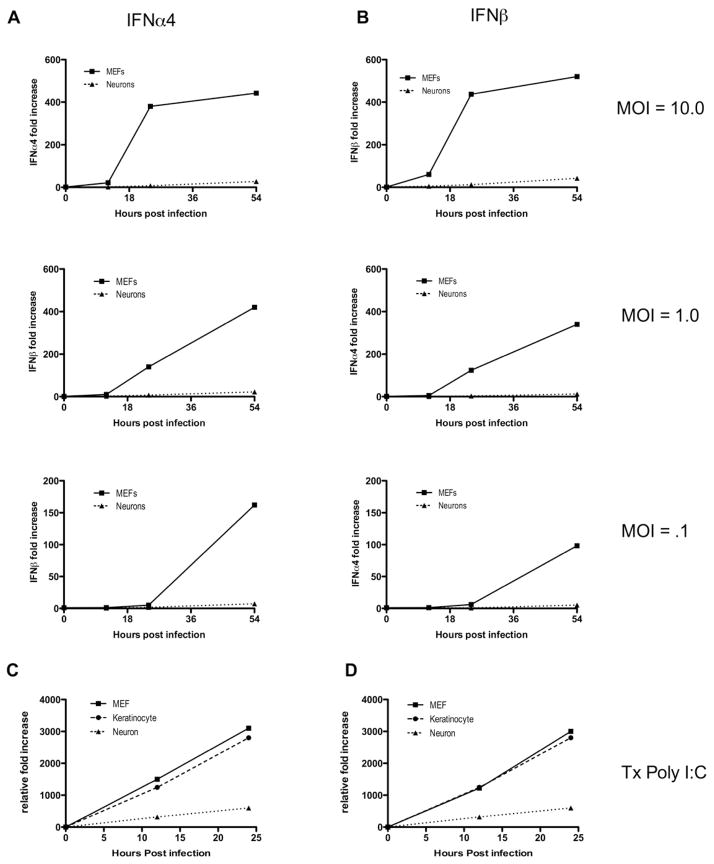
To evaluate the innate HSV antiviral pathway in controlling infection in different cell types, we first sought to evaluate the ability of different HSV-1 target cells to induce type I IFN production upon viral infection. To this end, we infected neurons, keratinocytes and mouse embryonic fibroblasts (MEFs) with various multiplicity of infection (MOI) of HSV-1 and examined IFN-α4 and IFN-β mRNA expression by RT-qPCR. As expected, both IFN-α4 and IFN-β were induced in a time-dependent manner in MEFs at different MOIs (Figure 1A & B). In contrast, dorsal root ganglion (DRG) neurons failed to induce significant levels of IFN mRNA at all MOI’s tested (Figure 1A & B), and consequently failed to induce expression of the ISG, Mx-1 (Figure S1A). In addition, while a slight reduction in viral titers at later time points was observed in infected neurons compared to MEFs (Figure S1B), the near complete absence of IFN-α4 and IFN-β in the HSV-infected neurons could not be accounted for by the moderate reduction in viral titers.
Figure 1. Neurons are impaired in their capacity to respond to viral stimulation and induce type I IFNs.
(A, B) Mouse embryonic fibroblasts (MEFs) and neurons were infected with HSV1-GFP at MOIs of 0.1, 1.0 and 10.0. RNA was isolated from all cells at 0, 12, 24, and 54 hours post-infection. Relative fold increases in IFN-α4 (A) and IFN-β (B) production were determined by quantitative RT-PCR of isolated RNA relative to the housekeeping gene hprt. (C, D) MEFs, keratinocytes, and neurons were transfected with Poly I:C (1.0 μg/mL). RNA was isolated from all cells at the time points indicated, and fold increases of IFN-α4 (C) and IFN-β (D) relative to hprt were determined by RT-qPCR. Data are representative of three independent experiments. See also Figure S1.
To rule out the possibility that observed differences were due to viral inhibition of IFNs specifically in neurons, these cell types were transfected with Poly I:C, a synthetic mimic of a viral pathogen associated molecular pattern (PAMP) that activates MDA5-dependent cytosolic nucleic acid sensing pathway (Gitlin et al., 2006; Kato et al., 2006). Interestingly, we found that although significant levels of type I IFNs were induced in all cell types, the levels were strikingly lower in neurons than in the mitotic cell types tested (Figure 1C & D), despite the fact that Poly I:C transfection efficiency was comparable between these cell types (Figure S1C). These data indicate that neurons are limited in their ability to sense certain viral PAMPs and/or induce robust IFN gene expression.
Mitotic cells are more responsive to Type I IFN than neurons
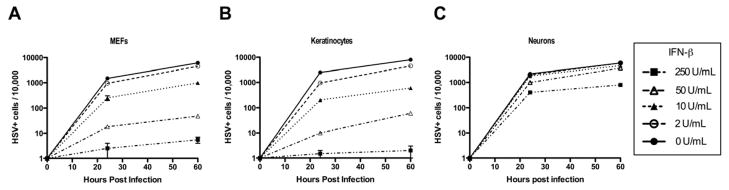
Multiple cell types contribute to type I interferon production during a viral infection (Stetson and Medzhitov, 2006). To examine whether neurons and mitotic cells respond equivalently to exogenous type I IFN treatment, both cell types were treated with increasing amounts of recombinant IFN-β. Eighteen hours later, IFN-treated neurons or mitotic counterparts were challenged with HSV-1-GFP and infected cells were enumerated by flow cytometry. Increasing doses of exogenous IFN-β dramatically reduced HSV-1 infection in both MEFs and keratinocytes (Figure 2A & B). In contrast, the same concentrations of IFN-β provided significantly less protection in neurons (Figure 2C). In order to confirm that IFN-β pre-treatment was significantly less effective in inducing an anti-HSV-1 state in neurons than in mitotic cells, we evaluated production of infectious HSV-1 following IFN-β pre-treatment. Although increasing doses of IFN-β significantly reduced viral titer in both MEFs and neurons, the extent of reduction was significantly greater in MEFs (Figure S2A). The near complete lack of HSV-1 infection in MEFs treated with 250 U/ml of IFN-β is likely due to a combination of robust antiviral ISG induction and death of infected MEFs (Figure S2B). Interestingly, infected neurons did not exhibit significant increase in cell death when treated with high concentrations of IFN-β. These data indicate that, while IFN-β induces an antiviral state in both mitotic cells and neurons, IFN-β confers less antiviral protection against HSV-1 in neurons than in mitotic cells.
Figure 2. Neurons have impaired type I IFN-dependent anti-HSV-1 protection.
(A–C) Primary keratinocytes, MEFs, and neurons were pre-treated for 18 hours with the indicated amount of IFN-β. Following pre-treatment, all cells were infected with 0.5 MOI of HSV1-GFP. At the time points indicated, cells were isolated and analyzed for GFP expression by flow cytometry. Data are shown as number of infected cells (GFP+) per 10,000 cell events for MEFs (A), keratinocytes (B), and neurons (C). Data are representative of four independent experiments. See also Figure S2.
Type I IFN fails to prime neurons for cell death
One major mechanism whereby type I interferon prevents viral replication is by inducing cell death in infected cells. We speculated that the reduced effectiveness of type I IFN in limiting viral replication might be due to reduced IFN-triggered cell death in neurons. As neurons are post-mitotic, non-renewable cells essential for host survival, reduced responsiveness to type I IFN observed in neurons might serve to help to minimize neuronal death and thereby enhance host survival. To directly test cell type specific sensitivity to IFN-induced death, we evaluated cell death in IFN-β-pre-treated MEFs and neurons challenged with Poly I:C. Type I IFN pre-treatment of fibroblasts primes them for execution by apoptosis upon detection of viral replication intermediates (Esaki et al., 2010). Consistently, we found that pre-treatment with IFN-β profoundly increased cell death in Poly I:C-transfected MEFs (Figure 3A). We observed a similar relationship in Poly I:C-transfected keratinocytes (data not shown), indicating type I interferon primes cell death upon detection of viral replication intermediates in mitotic cell types. In contrast, neurons were completely resistant to the effects of IFN-β for cell death upon Poly I:C stimulation (Figure 3A). Resistance to cell death was not a general feature of neurons, since they succumbed to apoptotic cell death upon induction of apoptosis by an IFN-independent pathway through staurosporine treatment (Figure 3B).
Figure 3. Type I interferon primes mitotic cells, but not neurons, for cell death.
(A) MEFs, and neurons were pre-treated for 18 hours with the indicated amount of IFN-β. All cells were then transfected with Poly I:C (1.0 μg/mL) for one hour. At the time points indicated, MEFs and neurons were isolated, stained with trypan blue and viable cells were counted by hematocytometer (top panel). MEFs and neurons were isolated, stained with Annexin V and 7-AAD, and analyzed by flow cytometry (bottom panel). Data are representative of three independent experiments. (B) MEFs and neurons were treated with staurosporine and isolated, stained with trypan blue, and counted by hematocytometer at the time points indicated. Error bars represent SEM. See also Figure S3.
Next, we wished to interrogate the mechanism by which neurons are resistant to both exogenous type I IFN and to IFN-induced cell death. To this end, neurons and mitotic cells were stimulated with IFN-β and ISG mRNA expression levels were analyzed over a time course. Although neurons were capable of increasing the expression of ISGs in response to IFN-β, we observed lower steady state and IFN-induced mRNA levels of several key effector ISGs involved in host cell death, such as PKR, in neurons compared to mitotic cells (Figure S3A). In contrast, we observed comparable levels of STAT1 and only a minor decrease in IRF-7 levels in neurons compared to MEFs (Figure S3A). HSV-1 interferes with IFN signaling at multiple levels (Chee and Roizman, 2004). Thus, we tested whether ISG levels following HSV-1 infection of cells treated with IFN-β are differentially regulated in neurons and MEFs. Our data indicated that HSV-induced decline in ISG expression was more exaggerated in neurons compared to MEFs (Figure S3C). Thus, the type I IFN-induced gene expression prior to and following HSV-1 infection are significantly attenuated in neurons, and could in part explain their impaired resistance to HSV-1 infection following IFN treatment.
Differential expression of ISGs in neurons compared to other cell types prompted us to evaluate whether type I IFNs induces robust antiviral protection against certain viruses but not others. To this end, we infected IFN-β-pre-treated neurons and MEFs with vesicular stomatitis virus (VSV) expressing G protein fused with GFP (VSV-GFP) (Dalton and Rose, 2001) and assessed the extent of viral infection by flow cytometry. In contrast to HSV-1, IFN-β pretreatment effectively restricted VSV infection in both neurons and MEFs (Figure S3B), suggesting that ISGs that control VSV infection are sufficiently induced by IFN in neurons. Collectively, these data indicate that type I IFN receptor signaling leads to altered expression of ISGs in the DRG neurons, leading to impaired control of some, but not all, viral pathogens.
Absence of autophagy in epithelial cells does not impact HSV mucosal defense
Upon identification of differences in the type I IFN response in neurons versus mitotic cells, we wished to investigate the role of autophagy in neuronal innate antiviral defense. Autophagy is an antiviral defense mechanism that does not require cell death for virus control. Autophagy has been shown to play a direct role in control of viral replication by engulfing cytosolic virions for lysosomal degradation (Levine, 2005). We utilized a mouse model of genital herpes infection to evaluate the contribution of autophagy in epithelial cells and neurons. In this model, HSV-1 introduced to the vaginal canal undergoes a primary round of replication in the mucosal epithelial cells at the site of inoculation. Subsequently, innervating neurons become infected, resulting in neuropathogenesis and death in a certain percentage of hosts. To determine the antiviral function of autophagy in epithelial cells, we generated Atg5 flox/flox x Krt14-cre Tg (KC-Atg5 KO) mice to specifically delete Atg5 within the keratinocytes (KC). Selective deletion of Atg5 in epidermal keratinocytes at transcriptional and functional levels was confirmed (Figure 4A–D). The level of Atg5 mRNA expression was significantly lower in the keratinocytes isolated from the KC-Atg5 KO mice but not in the liver cells (Figure 4A, C). In addition, measurement of LC3-I and its autophagosome associated form (LC3-II) in primary cells isolated from the KC-Atg5 KO mice by Western blot (Klionsky et al., 2008) revealed a selective defect in LC3-II formation in the keratinocytes but not in the liver cells (Figure 4B, D). Adult female KC-Atg5 KO mice and Atg5 flox/flox x Krt14-cre(−) littermates (WT) were intravaginally infected with WT HSV-1. We found no difference in viral replication within the vaginal mucosa in the absence of autophagy in the keratinocytes (Figure 4E). Furthermore, cytokine secretion in the vaginal mucosa was indistinguishable between KC-Atg5 KO mice and WT counterparts (Figure S4). Collectively, these results indicated that autophagy in epithelial cells does not significantly contribute to local inflammatory response or antiviral defense in HSV-1 infection.
Figure 4. Viral inhibition of autophagy in an infected, non-epithelial cell type is required for HSV-1-induced disease.
(A–D) Keratinocytes and total liver were isolated from ATG5flox.flox Krt14cre+ (KO) and ATG5flox.flox Krt14cre- (WT) mice. ATG5 mRNA expression relative to levels in KO is shown for keratinocytes (A) and liver (C). Western blot analysis of LC3-I and LC3-II from isolated keratinocytes (B) and total liver (D) is shown. Loading control (β-actin) for each sample is shown below. n > 2 for all genotypes and data are representative of two independent experiments. (E–H) Depo-provera treated Atg5 flox/flox x Krt14-cre Tg (KC-Atg5 KO) and Atg5 flox/flox Krt14cre(−) (WT) littermates were intravaginally infected with 5,000 pfu of either HSV-1 bbd or the rescue virus bbdR. (E) Vaginal washes were collected daily and viral titers were measured. Mice were weighed (F), clinically scored (G), and monitored for survival (H) every 24 hours post infection. Error bars represent SEM of n > 12 mice per group. **p < .001; ***; p < .0005 mortality of bbdR infected mice relative to bbd infection. Data are pooled from three independent experiments. See also Figure S4.
HSV encodes a virulence factor, ICP34.5, which inhibits autophagy through binding to Beclin-1 and preventing de novo autophagosome formation (Orvedahl et al., 2007). Therefore, it was possible that a potential contribution of epithelial cell autophagy to antiviral immunity was being masked by ICP34.5-dependent inhibition of autophagy in wild-type mice. To address this possibility, wild-type mice were infected intravaginally with either a mutant HSV-1 in which the Beclin-1 binding domain of ICP34.5 has been specifically deleted (bbd) or with the rescued virus (bbdR) (Leib et al., 2009). Viral replication, peak viremia, and viral clearance in the genital mucosa were indistinguishable after infection with bbd and bbdR, indicating viral replication in the epithelial cells was independent of the ability of HSV to inhibit autophagy (Figure 4E). Local cytokine production was also similar between infection with bbd and bbdR, confirming our previous observations that the absence of autophagy in the vaginal epithelium does not significantly impact the innate or adaptive immune cytokine responses (Figure S4). Collectively, these data indicated that autophagy was not required for HSV-1 control within the vaginal epithelium.
HSV inhibition of autophagy in non-epithelial cell type is required for HSV induced mortality
Next, we examined whether autophagy in the context of non-epithelial cells is required for control of HSV-1 infection. To this end, we intravaginally infected KC-Atg5 KO mice and Atg5 flox/flox x Krt14-cre(−) (WT) mice with either bbd or bbdR virus and evaluated disease outcome. We found a striking difference in pathology, weight loss, and survival between mice infected with bbd and bbdR virus (Figure 4F–H). Approximately 50% of all mice infected with bbdR (WT HSV-1) developed advanced clinical manifestations and ultimately succumbed to infection. In contrast, mice infected with the bbd mutant HSV-1 were completely resistant to viral induced pathology or mortality. Importantly, differences in morbidity and mortality were entirely independent of autophagy in epithelial cells, as nearly identical percentages of KC-Atg5 KO mice and WT mice succumbed to infection by bbdR (Figure 4F–H). Collectively, these data indicated that autophagy was critical in controlling HSV-1 disease in a non-epithelial cell type in vivo.
Attenuation of bbd mutant HSV-1 is not dependent on enhanced T cell responses
Autophagy has been shown to enhance MHC class II antigen presentation of cytosolic viral antigens in professional APCs and in non-professional APCs such as epithelial cells (Dengjel et al.; Leib et al., 2009; Paludan et al., 2005; Schmid et al., 2007). Thus, it was possible that pathogenesis of the WT HSV-1 is due to impaired activation of adaptive immune responses by infected APCs. To evaluate this possibility, we examined CD4+ T cell responses in the draining lymph node following infection with bbd and bbdR, as Th1 cells play a key role in viral control in HSV-1 infected mice (Harandi et al., 2001; Milligan et al., 1998). At six and eight days post infection, lymph node enlargement, total CD4+ T-cell number, and CD4+ T-cell IFN-γ production in response to viral antigen stimulation was comparable between mice infected with bbd and bbdR (Figure 5A–C). These data indicated that viral inhibition of host autophagy does not significantly alter the generation of mucosal CD4+ T cell responses. In addition, infection with bbd or bbdR HSV-1 showed identical viral replication in the vaginal mucosa of both CD4 (Figure 5D & E) and CD8 (Figure 5F & G) deficient mice. Infection of Cd8−/− mice with bbdR resulted in mortality equivalent to infection of wild-type mice, while no morbidity or mortality was observed in infection with bbd. Similarly, bbd infection of CD4-deficient mice did not result in mortality or any clinical manifestations. In contrast, infection of Cd4−/− mice with bbdR resulted in 90% mortality (Figure 5E), consistent with previous reports demonstrating the critical role for CD4+ T cells in HSV control (Harandi et al., 2001; Iijima et al., 2008; Milligan et al., 1998). These data indicated that, unlike corneal infection (Leib et al., 2009), the differences in disease outcome upon vaginal infection with bbd and bbdR were not dependent on CD4 or CD8 T-cell responses.
Figure 5. Neuro-attenuation of bbd is independent of T-cell responses.
(A–C) Depo-provera treated WT C57BL/6 mice were intravaginally infected with 20,000 pfu of HSV-1 bbd or the rescue mutant bbdR, and were analyzed on days six and eight post infection. Total number of lymphocytes (A), and CD4+ T-cells (B) from the iliac and lingual draining lymph nodes is depicted. (C) Isolated CD4+ T-cells were co-cultured with naïve splenocytes in the presence of heat-inactivated HSV-1. IFN-γ secretion from CD4+ T cells was measured by ELISA. (D–G) CD4−/−, CD8−/−, and WT control C57BL/6 mice were intravaginally infected with 2,000 pfu of the indicated strain of HSV-1. Vaginal washes of CD4−/− (D) and CD8−/− (F) mice were collected daily and viral titers were measured in Vero cells. CD4−/− (E) and CD8−/− (G) mice were monitored for survival every 24 hours post infection. Error bars represent SEM of n > 5 mice per group. Data are pooled from four (A–C) or three (D–F) independent experiments. See also Figure S5.
In addition to binding Beclin-1 and inhibiting autophagy, ICP34.5 binds to TBK1 and blocks the induction of antiviral genes (Verpooten et al., 2009). TBK-1 binding is mediated by the region that partially overlaps with that deleted in the bbd mutant. To evaluate if bbd deletion in ICP34.5 results in loss of blockade of type I IFN induction, MEFs and neurons were infected with either bbd or bbdR, and IFN-β expression levels were examined. As shown in Figure S5, we observed a comparable increase in IFN-β expression levels in bbd-infected MEFs or neurons over the bbdR-infected counterparts. These data are consistent with a previous report showing that IRF3 deficiency fails to restore virulence of bbd mutant HSV-1 in vivo (Leib et al., 2009). Therefore, these data argue that the avirulent phenotype of the bbd HSV-1 mutant is unlikely explained by the lack of TBK-1 inhibition. However, it is possible that bbd mutation results in additional defects that could contribute to the attenuation we observe in vivo.
Autophagy is required to restrict viral replication in the peripheral nervous system
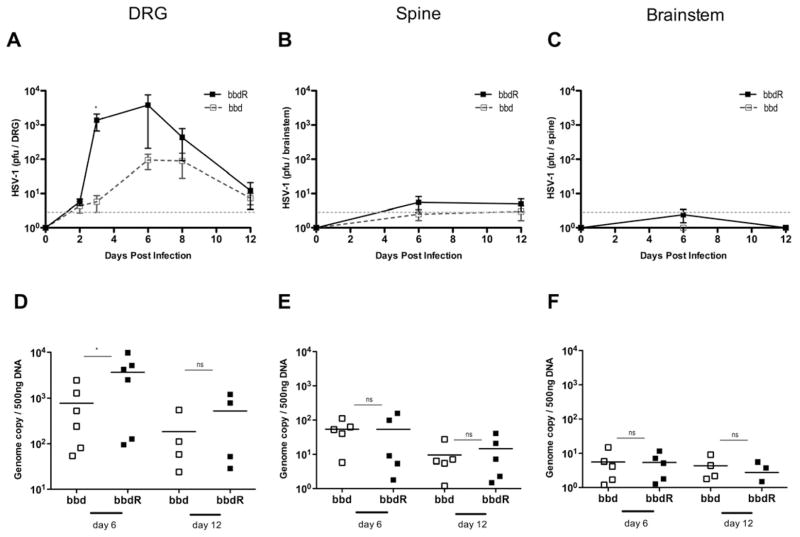
Our data indicated that while autophagy was dispensable for viral control in epithelial cells, it functioned in a critical capacity in another infected cell type. Given that HSV-infection is primarily limited to mucosal epithelial cells and neurons (Iwasaki, 2003; Parr et al., 1994; Parr and Parr, 2003), we hypothesized that autophagy is critical in antiviral defense in neurons but not epithelial cells. To test this hypothesis, we first examined the ability of bbd and bbdR viruses to replicate in the DRG innervating the vaginal mucosa in vivo. Wild type C57BL/6 mice were intravaginally infected with either bbd or bbdR HSV-1 and innervating DRG were isolated at several time points following infection. Infectious virus of both strains was detectable at 48 hours post infection, suggesting viral entry to the PNS occurred at approximately the same time (Figure 6A). However, viral replication was markedly higher in mice infected with bbdR at later time points, suggesting that autophagy within neurons limits HSV-1 viral replication in the absence of viral evasion.
Figure 6. Autophagy is required to restrict neuronal HSV-1 replication.
Depo-provera treated wild-type C57BL/6 mice were infected with 2 × 104 pfu of the indicated strain of HSV-1. Dorsal root ganglia, spinal cord, and brainstem were isolated at the time points indicated, homogenized. Viral titers were measured in Vero cells (A–C) and genome copy of isolated DNA was determined by qPCR (D–F). Error bars represent SEM (A–C) of n > 5, n>4 (D–F). Gray dotted line indicates limit of detection. Data are pooled from three independent experiments. See also Figure S6.
Next, we evaluated whether morbidity and mortality we observed in bbdR-infected mice corresponded to an increase in HSV-1 replication in the nervous tissue. First, we analyzed the viral replication in the central nervous system at various time points, and found that neither bbdR nor bbd undergoes significant replication in the brain stem or the spinal cord of the infected animal (Figure 6B & C). Additionally, viral genome copy number was comparable between bddR and bbd in HSV-1 infected brainstem and spinal cord (Figure 6E & F). In contrast, both bbd and bbdR replicated in the DRG, and bbdR-infected mice tended to have elevated viral copy numbers in the DRG (Figure 6A & D). Next, we examined whether HSV-1 infection leads to inflammation and pathology in the PNS and CNS. H & E stained sections of the DRG, spinal cord, and brainstem from bbd- and bbdR- infected mice at various time points after ivag infection were analyzed in a blinded manner. Consistent with the minimal viral invasion observed in the CNS, we detected minimal inflammation in the CNS even with a high viral challenge dose (Figure S6). In contrast, the DRG from bbdR-, but not bbd-infected, mice had signs of inflammatory infiltrate and tissue damage (Figure S6). Collectively, these data indicate that mice infected with WT HSV-1 capable of inhibiting host autophagy succumb to a neurologic disease primarily restricted to the PNS.
Autophagy and type I IFN pathway restrict viral replication in parallel in PNS neurons
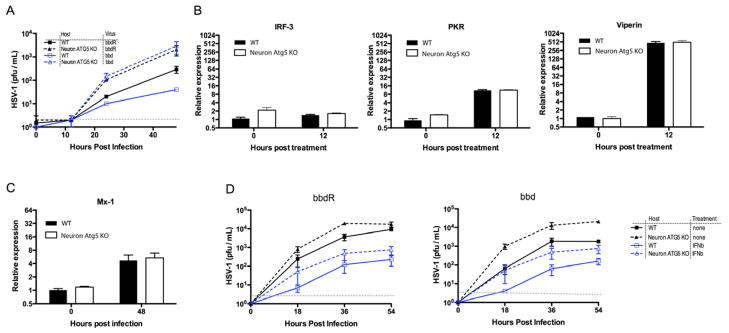
Our data demonstrated that an HSV-1 mutant incapable of inhibiting host autophagy (bbd) replicates similarly as the WT HSV-1 (bbdR) at the mucosal site of infection but is significantly impaired in replication in the PNS, thus implying that autophagy in neurons is critical in HSV defense. However, it is possible that the difference in host outcome we observed in infection with bbd and bbdR infection results from cell types other than epithelial cells or neurons. Therefore, to directly test the requirement for autophagy within neurons, we crossed Atg5 flox/flox mice with Nestin-cre Tg mice to conditionally delete autophagy in neurons. Neuron-specific deletion of autophagy results in accumulation of self-proteins and development of severe neurological impairments beginning at three weeks of age (Hara et al., 2006; Komatsu et al., 2006). In order to directly assess the requirement of autophagy in antiviral defense and minimize complications involving accumulation of host proteins, we isolated DRG neurons from 2.5-week-old Atg5 flox/flox x Nestin-cre Tg (+) (Neuron-Atg5 KO) and Atg5 flox/flox x Nestin-cre (−) (WT) littermate control and infected them ex vivo. HSV-1 viral replication was markedly increased in neurons in which autophagy had been conditionally deleted, regardless of the viral genotype (Figure 7A). Notably, Atg5-deficient neurons supported higher bbdR viral replication than the WT neurons, implying that autophagy mediates rapid antiviral defense within neurons even prior to the viral ICP34.5 gene expression. Importantly, Atg5-deficient neurons supported replication of bbd and bbdR to similar extent, indicating that the defect in bbd replication is restored in the absence of host autophagy gene.
Figure 7. Autophagy and type I IFNs restrict HSV-1 replication in neurons in parallel.
Primary neurons were isolated from Atg5 flox/flox x Nestin-cre Tg (Neuron Atg5 KO) and Atg5 flox/flox Nestin-cre(−) (WT) littermates. Neurons were infected at the indicated MOI with either bbd or bbdR HSV-1, at MOI of 0.01 (A), or at MOI of 0.1 with or without treatment with 250 U/ml of IFN-β 18 hours prior to infection (D). (A, D) Supernatants were collected at points indicated and viral titers were measured. (B & C) RNA isolated from neurons treated with 1,000 U/ml of IFN-β alone (B), or followed by HSV-1 infection for additional 48 hours was used to measure expression of indicated ISG by RT-qPCR. Error bars represent SEM and data are representative of two to four independent experiments. Gray dotted line indicates limit of detection. See also Figure S7.
The interferon pathway and certain Atg genes, including Atg5, have recently been shown to cooperate in innate antiviral defense (Hwang et al., 2012). Specifically, this study demonstrated that IFN-γ blocks norovirus replication by recruiting Atg proteins to inhibit the formation of replication complex. Therefore, we wished to determine if Atg5 and type I IFN pathways operate independently or cooperatively to restrict HSV-1 replication in neurons. To this end, we tested whether Atg5 is required for IFNαβR signaling and/or carrying out IFN-dependent antiviral effector functions in neurons. In order to evaluate whether Atg5 is required for IFNαβR signaling, we examined the expression levels of ISGs following treatment with IFN-β, with or without HSV-1 infection. We observed nearly identical ISG mRNA levels in WT and Atg5-deficient cells induced by IFN-β treatment alone (Figure 7B) or in combination with HSV-1 infection (Figure 7C). These data indicated that the IFN-αβR signaling in general or in the context of HSV-1 infection does not require Atg5 in neurons.
Next, to test whether Atg5 is required for IFN-dependent antiviral defense, we examined HSV-1 replication in Atg5−/− neurons following IFN-β treatment. If Atg5 is required for antiviral effects of type I IFNs, exogenous IFN-β would not be expected to protect Atg5−/− neurons from HSV-1 infection. However, our data demonstrated that IFN-β conferred significant protection in Atg5−/− neurons (Figure 7D). Of note, both bbdR and bbd still replicated at higher levels in Atg5−/− neurons than in WT neurons even after engagement of full ISG responses following IFN-β treatment, highlighting that autophagy provides additional antiviral defense in neurons. In contrast, in vitro replication of bbd and bbdR was indistinguishable in WT or Atg5 deficient MEFs whereas type I IFN treatment was sufficient to prevent viral replication in this cell type (Figure S7 and (Alexander et al., 2007)). Thus, both IFN and autophagy work in parallel to confer antiviral protection against HSV-1 in neurons, and ICP34.5 permits efficient HSV-1 replication by effectively countering both of these neuronal restriction strategies.
Discussion
Neuron death, engendered by either pathogen infection or lytic immune responses, can have severe pathological repercussions (Brot et al., 1997). Neurons employ alternative adaptive immune strategies to minimize cell death during infection (Joly et al., 1991; Levine et al., 1991). Here, we contribute to this paradigm by presenting evidence that neurons have reduced type I IFN dependent innate responses and are heavily reliant on an alternative, pro-survival pathway for antiviral defense. Our results provide experimental evidence for the hypothesis that autophagy is a particularly suitable means of antiviral defense used by neurons (Alexander and Leib, 2008; Orvedahl et al., 2007; Orvedahl and Levine, 2008), by promoting both cell survival and aiding in viral clearance. We found that HSV-1 infected neurons produced significantly less endogenous type I IFNs and entered a less effective anti-HSV-1 state in response to type I IFN stimulation relative to primary mitotic cells. In addition, neurons were resistant to type I IFN priming of cell death upon detection of viral PAMPs. Consistent with these observations, we found type I IFN induced a unique ISG expression pattern in neurons following IFN-β stimulation. In order to understand how neurons might compensate for attenuation of this key antiviral response pathway, we investigated the role of alternative forms of host innate defense. We found that either genetic ablation or viral inhibition of host autophagy resulted in significantly higher levels of HSV-1 replication in neurons, but not in mitotic cells in vitro and in vivo. Further, we demonstrate that autophagy and type I IFNs operate in parallel to defend against HSV-1 infection. Finally, our in vivo data suggest that even in the presence of intact innate and adaptive immune responses to the virus, neurons rely on cell intrinsic autophagy-dependent antiviral effector mechanism to counter HSV-1 infection.
Vertebrate antiviral defense mechanisms have evolved to depend heavily on the type I IFN system. Our data suggest a model in which the type I IFN-mediated viral control predominates over direct autophagic viral clearance in vertebrate tissues that can be readily repopulated by stem cells. However, in terminally differentiated, irreplaceable cell types such as neurons, we propose that the reduction in IFN responsiveness and cell death results in an enhanced dependency for non-lytic antiviral defense mechanisms such as autophagy. In support of this model, we find that type I IFN is less effective at blocking HSV infection and in priming cell death in neurons versus mitotic cells. These findings complement previous reports demonstrating neurons are resistant to certain adaptive, lytic immune responses (Joly et al., 1991; Knickelbein et al., 2008) and are less likely to undergo apoptosis following infection with HSV (Esaki et al., 2010).
Previous studies have demonstrated that type I IFNs are effective in controlling viral replication in neurons in vivo and in vitro, particularly in the central nervous system (Paul et al., 2007). For example, type I IFN pathway in neurons is critical in antiviral defense against HSV (Carr et al., 2003; Leib et al., 1999; Menachery et al., 2010), as well as numerous other neurotropic viruses (Iannacone et al., 2010; Lenschow et al., 2007; Samuel and Diamond). However, our data suggest that type I IFN responses in neurons are distinct from those in other cell types, such that they limit robust signaling capable of priming large-scale neuron death during viral infection, particularly in post-mitotic peripheral neurons. These findings are in agreement with a recent study demonstrating that cortical neurons have significantly lower basal ISG expression versus macrophages (Daffis et al., 2007) and that previously observed type I IFN-dependent protection against certain neurotropic viruses may be dependent on responses within astrocytes and other resident mitotic cells of the nervous system (Reinert et al., 2012). We speculate that such a mechanism serves as a ‘fail-safe’ for type I IFN signaling in order to avoid widespread death of neurons upon detection of viral infection. However, such a safety mechanism comes at a significant cost - reduced innate protection against certain neurotropic viruses.
We sought to determine how neurons might offset this “cost” in antiviral defense. We found that neurons rely on autophagy to limit HSV-1 replication. Even when ISGs are fully turned on by high levels of type I IFN, Atg5-dependent process confers additional antiviral defense in neurons. Intriguingly, the current evidence for the role of autophagy in direct antiviral defense in vertebrate in vivo (Orvedahl et al., 2007; Orvedahl et al., 2010) has been limited to nervous system infections of neurotropic viruses. In addition, other neurotropic viruses are known to interfere with autophagy in vitro (Chaumorcel et al., 2008). Based on our current study, we hypothesize that these findings reflect a unique neuronal requirement of autophagy in direct antiviral defense. However, it is important to note that viruses that are not known to be primarily neurotropic, including HIV-1 (Zhou and Spector, 2008), SIV (Alirezaei et al., 2008) and gamma-herpesviruses (Ku et al., 2008; Pattingre et al., 2005) also encode factors that specifically interfere with autophagy, suggesting that non-neuronal cell types also utilize autophagy to counter certain viral infections.
Our study demonstrates that the vertebrate immune system may utilize distinct innate antiviral strategies that are suitable for the physiology of the infected cell types. These findings not only reinforce the concept that vaccine and therapeutic treatment of viral infections should be tailored to maximize cell type-appropriate defense mechanisms but also provide important implications for treatment of HSV infections. Specifically, pharmacological inducers of autophagy might be beneficial in limiting replication of HSV-1, and possibly HSV-2, in neurons in both acute and chronic infections. Since our data indicated that PNS neurons have the capacity to limit virus infection through autophagy even in the face of ICP34.5-intact wild type HSV-1 infection, enhancing autophagic flux in neurons may hold promise in treating clinical infections in humans. In addition, similar approaches might be beneficial in treatment of other neurotropic or post-mitotic viral infections.
Experimental Procedures
Animals
Six week-old female C57BL/6 mice were obtained from National Cancer Institute (Frederick, MD). Atg5 flox/flox (Hara et al., 2006) (kind gift of Dr. Noboru Mizushima, Tokyo Medical and Dental University, Japan), Krt14-Cre Tg, Nestin-Cre Tg, Cd4−/−, and Cd8−/− (The Jackson Laboratory) mice were bred in the Yale animal facility. All procedures performed in this study complied with federal guidelines and institutional policies set by Yale Animal Care and Use Committee.
In vivo Infections
HSV-1 bbd and HSV-1 bbdR are previously described (Leib et al., 2009). HSV-1-GFP was a kind gift from Drs. P. Desai and S. Person (Johns Hopkins University, MD). HSV-1 was maintained, propagated, and tittered in Vero cells. Intravaginal HSV-1 infection was performed as described previously (Lee et al., 2010). Briefly, HSV-1 in 10 μL PBS was introduced into the vaginal canal of Depo-provera treated female mice. Following infection, vaginal washes were collected daily in 50 μl PBS. Mice were weighed and clinically scored every 24 hours post infection. Clinical score was assigned according to the following scale (Morrison et al., 1998): All mice were euthanized prior to reaching moribund state due to humane concerns. Viral titer from DRG was determined by removing the lumbar and sacral DRG from intravaginally infected mice. Isolated DRG was homogenized by rotor-stator and sonicated to release membrane bound virus.
Primary Neuron Isolation and Culture
DRG neurons were isolated from either adult (6–8 week old) C57BL/6 or juvenile (2–3 week old) Atg5 flox/flox Nestin-Cre Tg mice and Atg5 flox/flox Nestin-Cre(−) littermate controls (age and sex matched), as described previously (Malin et al., 2007). Briefly, DRG were removed from the spinal column, gently dissected by papain (Worthington), collagenase II (Worthington), dispase II (Roche), and gently triturated to a single cell suspension. Cells were then adhered to laminin (Sigma)/poly-D-lysine (Sigma) coated coverslips and cultured in Neurobasal media (Gibco) supplemented with B-27 supplement (Gibco), L-glutamine (Gibco), and neurite growth factor 2.5S (Millipore). The mitotic inhibitor 5-fluorodeoxyuridine (MP Biomedicals) and uridine (MP Biomedicals) were added to prevent outgrowth of mitotic cells.
Poly I:C Stimulation of Cells
MEF, neurons, and keratinocytes were stimulated with 1.0 μg/mL Poly I:C or Poly I:C Rhodamine (Invivogen) complexed to Lipofectamine 2000 (Invitrogen). All cells were transfected for one hour in Optimem media (Gibco). Where noted, cells were stimulated with 10.0 μg/ml staurosporine. At the indicated time points, adherent and detached cells were isolated by TrypLE (Gibco). The number of live cells was determined by trypan blue exclusion assay. Cell death was evaluated by staining all cells with 7-AAD and PE-Annexin V, according to the manufacturer’s instructions of the PE Annexin V Apoptosis kit (BD Pharmingen) by flow cytometry.
Statistics
Statistical analysis of survival curves was performed using the logrank (Mantel-Cox) test. Graphs containing two data sets were analyzed by unpaired t-tests while those containing more than two data sets were analyzed by one-way ANOVA followed by Tukey test, unless otherwise noted. All statistical analyses were performed in Graphpad Prism v5.0.
Supplementary Material
Highlights.
Neurons produce less type I interferons (IFNs) than mitotic cells.
IFN-induced responses to herpes simplex virus type 1 (HSV-1) are reduced in neurons.
Type I IFN primes primary mitotic cells, but not neurons, for cell death.
Neurons, but not epithelial cells, require autophagy to control HSV-1 in vivo.
Acknowledgments
We thank E. Foxman for critical reading of the manuscript. This study was supported by grants from the NIH to DL (EY09083) and to AI (AI081884, AI054359, AI062428). B.Y. was supported by the NIH National Research Service Award (T32AI07019) from the Interdisciplinary Immunology Training Program in Yale University, Department of Immunobiology. A.I. is a recipient of the Burroughs Wellcome Investigator in Pathogenesis of Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DE, Leib DA. Xenophagy in herpes simplex virus replication and pathogenesis. Autophagy. 2008;4:101–103. doi: 10.4161/auto.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DE, Ward SL, Mizushima N, Levine B, Leib DA. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. Journal of virology. 2007;81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Host defense, viruses and apoptosis. Cell Death and Differentiation. 2001;8:113. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- Brot MD, Rall GF, Oldstone MBA, Koob GF, Gold LH. Deficits in discriminated learning remain despite clearance of long-term persistent viral infection in mice. Journal of NeuroVirology. 1997;3:265. doi: 10.3109/13550289709029467. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Al-khatib K, James CM, Silverman R. Interferon-beta suppresses herpes simplex virus type 1 replication in trigeminal ganglion cells through an RNase L-dependent pathway. J Neuroimmunol. 2003;141:40–46. doi: 10.1016/s0165-5728(03)00216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumorcel M, Souquere S, Pierron G, Codogno P, Esclatine A. Human cytomegalovirus controls a new autophagy-dependent cellular antiviral defense mechanism. Autophagy. 2008;4:46–53. doi: 10.4161/auto.5184. [DOI] [PubMed] [Google Scholar]
- Chee AV, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. Journal of virology. 2004;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Keller BC, Gale M, Jr, Diamond MS. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KP, Rose JK. Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology. 2001;279:414–421. doi: 10.1006/viro.2000.0736. [DOI] [PubMed] [Google Scholar]
- Danthi P, Pruijssers AJ, Berger AK, Holm GH, Zinkel SS, Dermody TS. Bid regulates the pathogenesis of neurotropic reovirus. PLoS Pathog. 2010;6:e1000980. doi: 10.1371/journal.ppat.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell host & microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki S, Goshima F, Katsumi S, Watanabe D, Ozaki N, Murakami S, Nishiyama Y. Apoptosis induction after herpes simplex virus infection differs according to cell type in vivo. Archives of Virology. 2010:1. doi: 10.1007/s00705-010-0712-2. [DOI] [PubMed] [Google Scholar]
- Fields BN, Knipe DM, Howley PM. Fields virology. 5. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res. 2010;47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. The Journal of general virology. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double- stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, et al. Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell host & microbe. 2012;11:397–409. doi: 10.1016/j.chom.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, Von Andrian UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. The Journal of experimental medicine. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. The role of dendritic cells in immune responses against vaginal infection by herpes simplex virus type 2. Microbes Infect. 2003;5:1221–1230. doi: 10.1016/j.micinf.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Khanna KM, Lepisto AJ, Hendricks RL. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 2004;25:230–234. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Ku B, Woo JS, Liang C, Lee KH, Hong HS, EX, Kim KS, Jung JU, Oh BH. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008;4:e25. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T cell responses. Journal of virology. 2009 doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. The Journal of experimental medicine. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, Osiak A, Levine B, Schmidt RE, Garcia-Sastre A, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci U S A. 2007;104:1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. Apoptosis in viral infections of neurons: a protective or pathologic host response? Current topics in microbiology and immunology. 2002;265:95–118. doi: 10.1007/978-3-662-09525-6_5. [DOI] [PubMed] [Google Scholar]
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. Journal of virology. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Menachery VD, Pasieka TJ, Leib DA. Interferon regulatory factor 3-dependent pathways are critical for control of herpes simplex virus type 1 central nervous system infection. Journal of virology. 2010;84:9685–9694. doi: 10.1128/JVI.00706-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication- defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 Confers Neurovirulence by Targeting the Beclin 1 Autophagy Protein. Cell host & microbe. 2007;1:23. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Autophagy and viral neurovirulence. Cell Microbiol. 2008;10:1747–1756. doi: 10.1111/j.1462-5822.2008.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, MacPherson S, Sumpter R, Jr, Talloczy Z, Zou Z, Levine B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell host & microbe. 2010;7:115–127. doi: 10.1016/j.chom.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Parr MB, Parr EL. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol. 2003;9:594–602. doi: 10.1080/13550280390246499. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89:770–778. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, Dagnaes-Hansen F, Ulhoi BP, Holm TH, Mogensen TH, Owens T, et al. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. Journal of virology. 2005;79:13350. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I Interferons in Host Defense. Immunity. 2006;25:373. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Verpooten D, Ma Y, Hou S, Yan Z, He B. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J Biol Chem. 2009;284:1097–1105. doi: 10.1074/jbc.M805905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Levine B. Autophagy genes in immunity. Nature immunology. 2009;10:461– 470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.