Abstract
BACKGROUND
Prostate-specific antigen (PSA) is a serine protease secreted as a zymogen. Previously, cell-free biochemical studies have identified various kallikreins (KLK) as candidate activating proteases. In this study, KLK2-mediated activation of PSA in cell-based in vitro, xenograft, and transgenic models was evaluated.
METHODS
Du145-derived PSA- or KLK2-expressing clones were coincubated in vitro and in vivo to evaluate KLK2-induced PSA activity. While mice possess orthologs of KLK4-15, they do not have functional orthologs of PSA or KLK2. Therefore, transgenic animals expressing PSA or both PSA and KLK2 were generated to assess orthotopic PSA activation.
RESULTS
PSA is activated by KLK2 when the cells are physically in contact, and through co-conditioned media. In vivo, the free (inactive PSA) to total (active + inactive PSA) ratio in the blood is decreased when PSA and KLK2-expressing cells are co-inoculated subcutaneously, suggesting increased active PSA. Additionally, double-transgenic mice expressing both genes in the prostate produce more active PSA compared to single transgenic animals. A longitudinal evaluation over a 2-year period demonstrated no morphologic changes (i.e., no PIN or prostate cancer) due to PSA or PSA/KLK2 double transgene expression relative to non-transgenic mice.
CONCLUSIONS
These data demonstrate, with biologically relevant models, that KLK2 is the protease responsible for activating PSA. While PSA is involved in the processing and release of a number of important growth factors, our results suggest that active PSA is not sufficient to induce the development of prostate cancer or prostate cancer precursors in aging PSA transgenic mice.
Keywords: kallikrein, zymogens, activation cascade
INTRODUCTION
Prostate-specific antigen (PSA) is used extensively to screen for prostate cancer, to detect recurrence following local therapies and to follow response to therapies. While much is known about PSA’s role as a biomarker, the exact physiologic role it plays in the normal prostate and in the pathobiology of prostate cancer has not been clearly defined. However, it is clear that prostate cancer cells, including those from the majority of metastatic sites from patients with poorly differentiated, castration resistant disease, continue to produce high levels of PSA that manifests as increasing serum PSA levels as the disease progresses [1].
Prostate cancer tumors also maintain the machinery required to process PSA to an enzymatically active form. PSA is synthesized as a zymogen or prepropeptide form that includes a 17 amino acid leader (pre)sequence removed intracellularly by signal peptidases, and a seven amino acid inhibitory (pro)sequence that is removed extracellularly. PSA must be correctly processed to become enzymatically active [2]. Under- and over-processing of the pro-peptide have been demonstrated and both result in the production of enzymatically inactive PSA. While the physiologic protease(s) responsible for this correct processing of pro-PSA to active PSA in vivo is unknown, the presence of arginine at the cleavage site suggests that pro-PSA is activated by an enzyme with trypsin-like substrate specificity. PSA is a member of the kallikrein (KLK) gene family (now termed KLK3) [3] and, since the normal prostate produces a number of kallikreins with trypsin-like activity, several studies have evaluated the ability of various kallikreins to correctly process PSA. Previously, recombinant KLK2 was shown to convert pro-PSA into active PSA in vitro, suggesting that KLK2 may be the physiologic PSA processing protease [4–6] However, previous analyses of the LNCaP human prostate cancer cell line demonstrated that both its endogenously produced PSA and KLK2 are predominantly in their pro forms [7]. Previously we demonstrated that only 15–20% of the PSA produced by the LNCaP cell line is active in serum-free media [8]. These results suggest that either this processing is KLK2-dependent but occurs suboptimally in cell lines, or that other proteases may be involved in PSA processing. In this regard KLK4 [9], 14 [10], and 15 [11] can also activate PSA in vitro. PSA, therefore, may be activated by a number of kallikreins or non-kallikrein trypsin-like proteases that are preferentially expressed in prostate tissue. Alternatively, PSA activation may require a cascade of proteases for activation [12].
The goal of this study was to use co-culture, xenograft, and transgenic systems to clarify whether KLK2 is the physiologic PSA activator. Using the KLK2- and PSA-null human prostate cancer cell line, Du145, we generated clones stably expressing either PSA or KLK2. This cellular system shows that KLK2 can indeed activate PSA. Since the murine genome contains orthologs to all members of the human KLK family except PSA and KLK2 [13], we generated transgenic mice expressing these genes within the prostate. While the PSA isolated from the PSA mouse had little activity, crossing these animals with KLK2 transgenic mice resulted in increased production of active PSA within the prostate. These mice were subsequently used to perform longitudinal studies to determine whether expression of PSA and KLK2 in the prostates of aging mice could initiate prostate carcinogenesis.
MATERIALS AND METHODS
mRNA Isolation, cDNA Production, and qPCR
LNCaP and Du145 human prostate cancer cell lines (ATCC) were maintained in RPMI (Invitrogen) supplemented with 10% FBS (Invitrogen). Following growth to 70% confluence, the cells were washed with HBSS (Invitrogen) before the addition of Trizol (Invitrogen). The plates were then scraped and the material was then subjected to column-based RNA isolation (RNeasy, Qiagen). One microgram of total RNA was then used for cDNA generation with oligo dT primers (Super-Script III First-Strand, Invitrogen). One microliter of cDNA was mixed with iQ SYBR Supermix (Bio-Rad) and primers to a final volume of 25 µl. The primers, used to a final concentration of 200 nM, were 5′-GAACCAGAGGAGTTCTTGCG-3′ (forward) and 5′-CCCAGAATCACCCCCACAA-3′ (reverse) for KLK2, 5′-AGCATTGAACCAGAGGAGTTCT-3′ (forward) and 5′-CCCGAGCAGGTGCTTTTG-3′ (reverse) for PSA, 5′-GTACCACCCCAGCATGTTCT-3′ (forward) and 5′-GGCTTTTCCGAAAGACACAA-3′ (reverse) for KLK4, 5′-CCGCTACATAGTTCACCTGG-3′ (forward) and 5′-AGGTGTGAGGCAGGCGTAACT-3′ (reverse) for KLK11, 5′-CTTGGAGTGGATCAGGGAAA-3′ (forward) and 5′-TCCGTTCTGTTCCATGTCAG-3′ (reverse) for KL-K15, and 5′-TGAACGGGAAGCTCACTGG-3′ (forward) and 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse) for GAPDH. Thermocycling was run on the iCycler iQ Real Time Detection System (Bio-Rad). Threshold cycle calculations were used as indications of KLK expression and normalized to GAPDH.
PSA and KLK2 Expression Constructs
Both the PSA and KLK2 cDNAs were cloned into the pcDNA3.1+Zeo vector (Promega) downstream of a CMV promoter. A cationic lipid (TransFast, Promega) was mixed with the expression constructs and used to transfect the Du145 cells. These were then subjected to selective pressure with maintenance media containing zeocin (Invitrogen) at 0.5mg/ml. Surviving clones were isolated, expanded and characterized for their secretion of the respective KLK.
Immunoblotting
Forty micrograms of protein from conditioned media or mouse prostatic tissue protein extract was subjected to polyacrylamide gel electrophoresis (PAGE) on a 12% denaturing gel. This was followed by a PSA western of the resulting blot with a polyclonal rabbit anti-human PSA antibody that cross-reacted with KLK2 (Dako).
Enzymatic Activity Assays
Enzymatic activity assays were performed as previously described [14] using PSA or KLK2 immuno-concentrated from serum-free media conditioned by transfected Du145 cells. Enzymatic activity was assayed using a 40 µM solution of the fluorescent peptide substrate (Mu-HSSKLQ-AMC for PSA, Mu-GKAFRR-AMC for KLK2). For enzymatic activity assays of PSA derived from transgenic animals, mouse prostates were isolated and homogenized in RPMI, from which samples were used to determine total protein and PSA levels. Five hundred nanograms of PSA from tissue homogenates and comparative total protein levels from control animals were subjected to immunoprecipitation and incubated with the PSA-specific fluorogenic substrate as described above.
Tumor Xenograft Studies
Xenograft studies were performed as previously described [15] by subcutaneous injection of cells suspended in a 60% mixture of Matrigel Matrix (BD Biosciences) into the flank of 6-week-old male nude mice (n = 10). Four weeks post-inoculation, blood was collected by cardiac puncture and assayed for free and total plasma PSA levels.
Transgenic Animals
The PSA and KLK2 cDNAs were cloned into the pGL2-Basic vector (Promega) downstream of the composite (ARR)2 probasin (PB) promoter, previously demonstrated to target gene expression to the murine prostate at high levels [16]. The linearized constructs were provided to the Transgenic Animal Core Facility at The Johns Hopkins School of Medicine, who generated founder mice in a C57 B10.D2 background. Candidate animals were genotyped by PCR, and used for subsequent studies. Homozygous PSA animals were crossed with heterozygous KLK2 mice. Mouse prostates were isolated from 6-month-old animals.
Immunohistochemistry
PSA staining was performed as previously described [17]. Slides were then overlaid with a coverslip prior to evaluation under brightfield illumination to assess PSA distribution.
RESULTS
Generation of Du145-Derived Clones Expressing PSA and KLK2
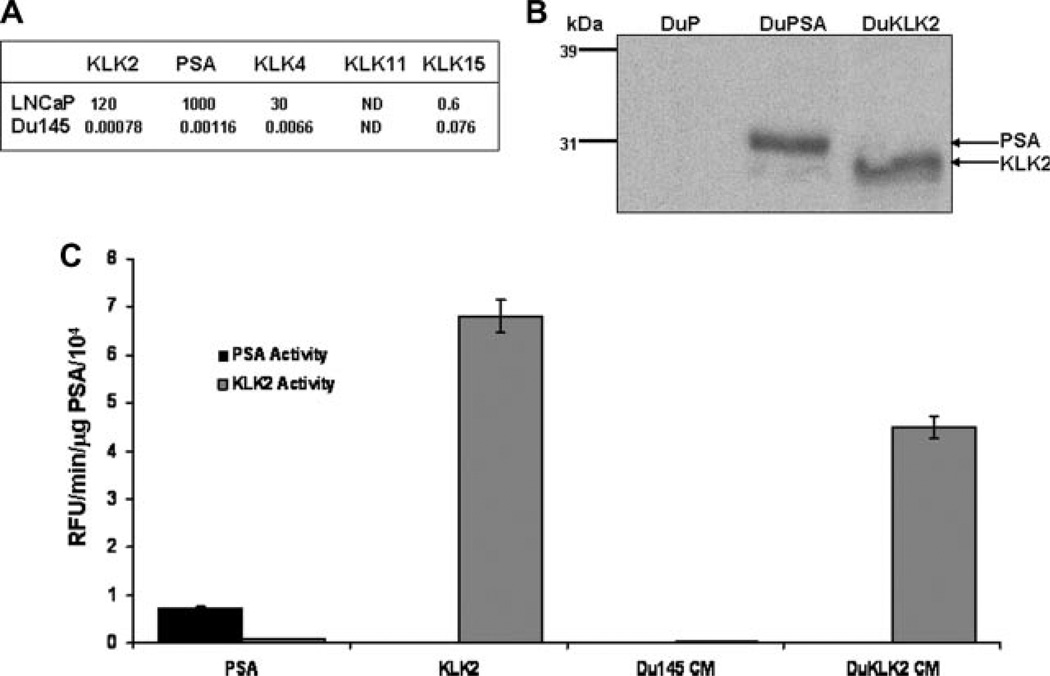
The Du145 human prostate cancer cell line lacks the androgen receptor and does not express PSA, KLK2, 4, 11, or 15 to any significant degree, while LNCaP cells make an abundance of PSA in addition to significant amounts of KLKs 2 and 4 (Fig. 1A). The expression of PSA or KLK2 isolated from serum-free conditioned media from transfected Du145 cells was confirmed by Western blotting (Fig. 1B). PSA and KLK2 immuno-precipitated from serum-free conditioned media from the respective clones was then assayed for enzymatic activity using previously described PSA [14] and KLK2 [18] substrates (Fig. 1C). The PSA-selective substrate was not cleaved by recombinant purified KLK2 (Fig. 1C). Overall, the activity of purified KLK2 with the PSA-selective substrate was significantly lower than activity of purified KLK2 with the KLK2-selective substrate. The KLK2 isolated from serum-free conditioned media of the KLK2-expressing Du145 cells had a high degree of activity against the KLK2 substrate.
Fig. 1.
PSA and KLK2-expressing Du145 clones. A: Relative mRNA levels of PSA and candidate activating KLKs in LNCaP and Du145 cells. B: Whole cell lysates of representative clones were used for Western blotting with an antibody that recognizes both PSA and KLK2. Compared to the vector clone (DuP), strong bands can be seen in lanes containing lysates from a PSA-expressing clone (DuPSA), and a KLK2-expressing clone (DuKLK2). Western blot of representative clones. C: Fluorogenic substrates cleaved selectively by PSA or KLK2. The PSA-specific substrate (Mu-HSSKLQ-AMC), or the KLK2-specific substrate (Mu-GKAFRR-AMC) were incubated with isolated PSA, rKLK2, or media conditioned by Du145 cells or KLK2-expressing Du145 cells. Experiments repeated in triplicate. Results presented as mean ± SE.
PSA Activation by KLK2 Co-Expression and Co-Culture
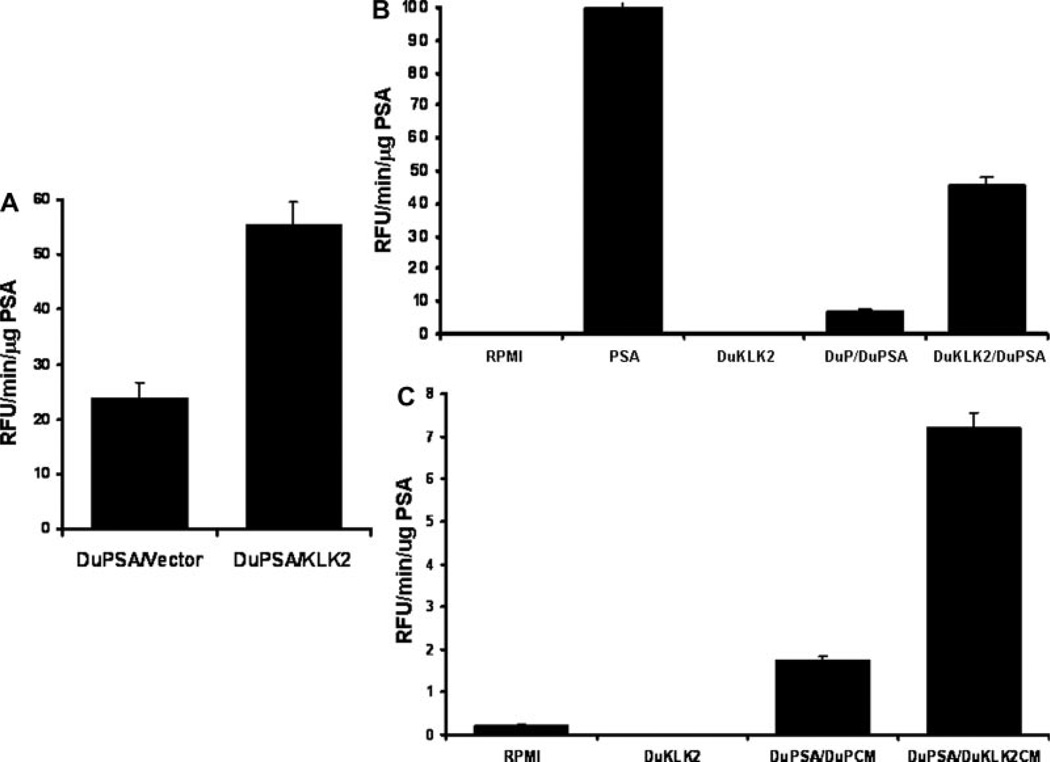
KLK2 was transiently transfected into Du145 cells already stably expressing PSA. The PSA isolated from serum-free conditioned media had twice as much activity as controls (Fig. 2A). Subsequently, mixing together of cells stably expressing PSA or KLK2 resulted in a sixfold increase in PSA activity (Fig. 2B). To determine if KLK2 activation was cell contact independent, media conditioned by KLK2-secreting cells was added to the PSA-secreting cells. The PSA activity in this context was markedly greater than any of the control conditions (Fig. 2C). Overall, these results demonstrate that, while KLK2 is secreted in an active form by these transfected Du145 cells, PSA is secreted in a predominantly inactive form but can become activated in the presence of active, soluble KLK2.
Fig. 2.
The effect of KLK2 on PSA activity in vitro. A: PSA-expressing Du145 -derived clones transiently expressing KLK2 display increased substrate cleavage activity. Cells stably expressing PSA were transiently transfected with either an empty vector or a KLK2 expression construct. B: PSA-expressing Du145 cells were mixed with KLK2-expressing clones or control cells before plating. C: PSA-expressing clones incubated in Du145 clone conditioned media. Media conditioned for 96 hr by KLK2-expressing cells (DuKLKCM) or vector controls (DuPCM) were added to PSA-expressing Du145 cells. Experiments repeated in triplicate. Results presented as mean ± SE.
In Vivo Co-Culture
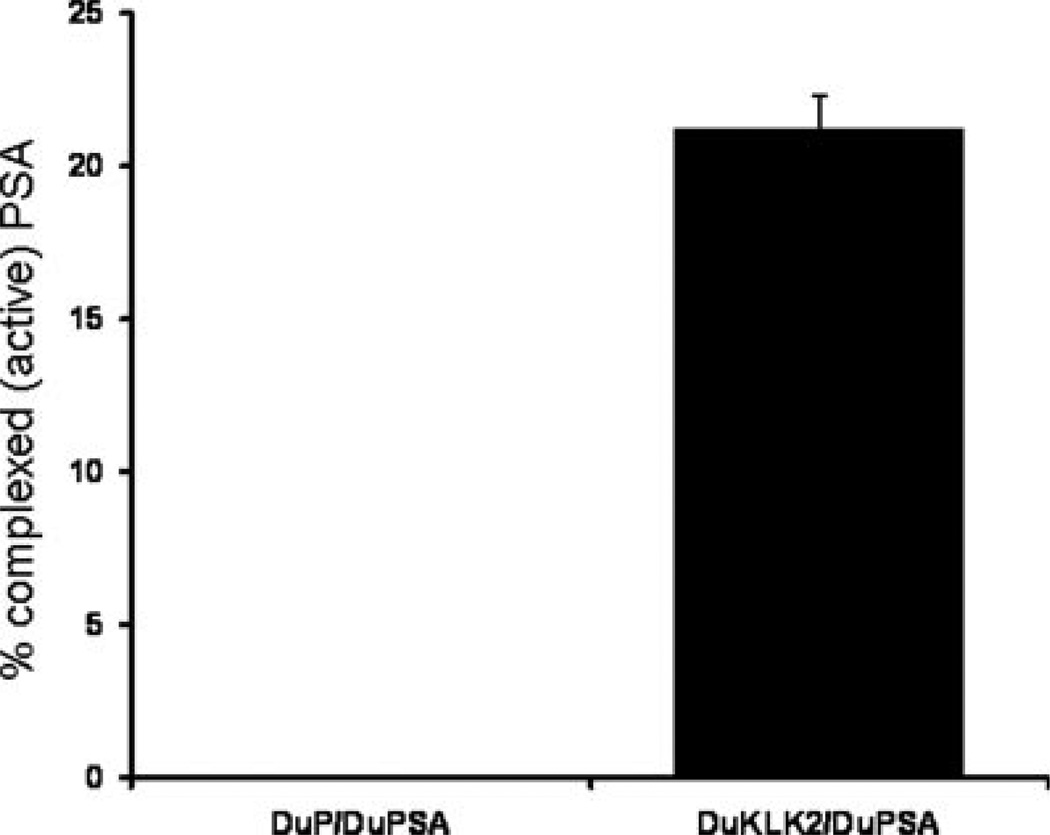
The results from these cell-based studies support earlier cell-free studies demonstrating that KLK2 can activate PSA. However, this KLK2 activation of PSA has never been evaluated in vivo. We took advantage of the fact that active PSA becomes complexed with serum protease inhibitors upon entering the circulation. We measured the difference between total (inactive- + active complexed) and free (inactive) PSA in the blood of tumor-bearing animals as a surrogate for the amount of active PSA entering the blood from the xenografts. Using this approach, we established chimeric xenografts of Du145 consisting of the same clonal mixtures used for the in vitro studies. After four weeks of growth, the plasma from these chimeric tumor-bearing mice was subjected to total and free PSA analysis. The results showed that the control PSA-secreting tumor chimera produced PSA protein that was entirely free PSA, consistent with a lack of activation within the xenograft. In contrast, in PSA/KLK2 chimeric tumors levels of ACT-bound PSA reached over 20% of the total circulating PSA protein, consistent with PSA activation by KLK2 within the xenograft (Fig. 3).
Fig. 3.
Blood from mice bearing mixtures of Du145-derived clones. Xenografts of PSA-expressing cells mixed with either control cells or KLK2-expressing cells were established and left for 4 weeks. Plasma from these animals was tested for total and free PSA. ACT-bound (complexed) PSA was determined by subtracting free PSA from total PSA. Results presented as mean ± SE.
PSA/KLK2 Transgenic Mice
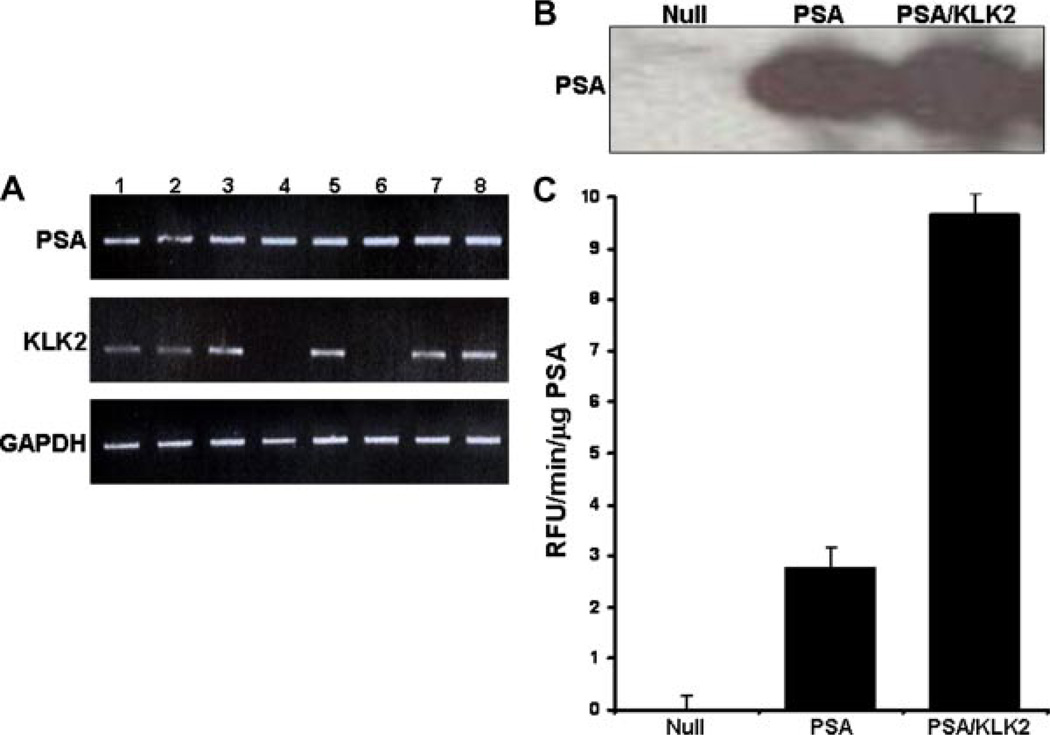
The above results demonstrate that enzymatically active PSA can be generated in human prostate cancer cells by KLK2 in a variety of cellular settings. The ability of KLK2 to activate PSA in the context of the normal prostate, however, requires a transgenic approach. Although mice express functional orthologs of KLK4-15, they do not have an ortholog of either PSA or KLK2. We used the (ARR)2 PB promoter to maintain prostate-specific transgene expression and successfully generated homozygous mice that produce PSA in their prostates (Fig. 4A,B). The PSA isolated from prostate tissue homogenates of these animals had low measurable levels of enzymatic activity compared to non-transgenic mice (Fig. 4C). To ask whether these mice would produce higher levels of enzymatically active PSA when co-expressed with KLK2, we generated KLK2 transgenic mice also using the (ARR)2 PB promoter and expression construct. KLK2 heterozygotes were crossed with homozygous PSA mice. Homogenates of ventral prostates from these mice had comparable total PSA levels (Fig. 4B) but revealed almost a fourfold increase in the PSA activity in the PSA/KLK2 transgenic animals (Fig. 4C).
Fig. 4.
PSA and PSA/KLK2 transgenic animals. A: Six-week-old candidate transgenic mice were genotyped by PCR for the incorporation of PSA (upper bands) or KLK2 (middle bands) cDNA. GAPDH cDNA levels (bottom bands) indicated equal loading. Lane numbers refer to individual genotyped animals. B: Prostatic tissue from non-transgenic (null), single PSA, or double PSA/KLK2 transgenic mice was used for Western blotting with the PSA/KLK2 cross-reactive antibody used previously. Western blot of representative mouse prostates. C: PSA extracted from transgenic mouse prostatic tissue was subjected to the PSA enzymatic activity assay as previously described. Experiments repeated in triplicate. Results presented as mean ± SE.
PSA Expression Does Not Initiate Cancer in the Transgenic Mouse Prostate
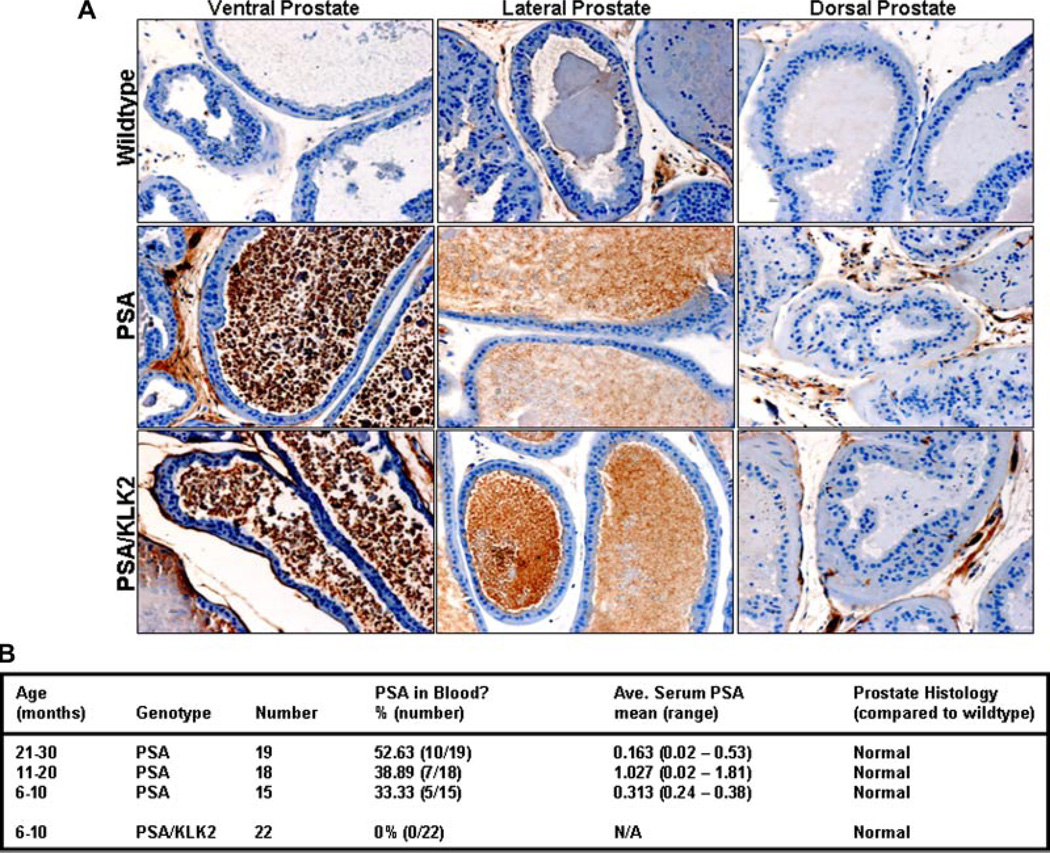
To study whether the production of PSA, active or inactive, in the mouse prostate was sufficient to initiate prostate carcinogenesis, transgenic animals expressing either PSA or PSA/KLK2 were sacrificed at varying time points (Fig. 5B). Animals were evaluated up to an age of 24 months based on the normal mouse life span of 2.5 years in order to encompass the full range of the life of the adult male. Overall, the majority of these aging transgenic animals did not have detectable PSA in the blood (Fig. 5B). A higher percentage of detectable PSA (i.e., ≥0.02 ng/ml) was observed in PSA transgenic, while none was detectable in the blood of the PSA/KLK2 group (Fig. 5B). This was despite comparable production of PSA in their prostates (Fig. 5A). No prostate pathology was observed in either the PSA or PSA/KLK2 mice (Fig. 5B).
Fig. 5.
Tissue and blood characteristics of transgenic animals. A: Immunohistochemical staining for PSA expression in the ventral, lateral and dorsal prostate lobes from representative 9 -month-old mice. Images from representative mouse prostates. B: Age-dependant blood PSA levels and prostate morphological features of transgenic mice. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Although the utility of PSA screening in the detection of prostate cancer continues to be the subject of much debate, PSA still remains the most important non-invasive indicator of the emergence and progression of prostate cancer. While thousands of papers have been written describing the use of PSA as a biomarker since its discovery more than 25 years ago, many questions remain unanswered regarding PSA, including its full function in the prostate, its repertoire of biologically relevant substrates, and the mechanisms whereby the inactive zymogen form is converted to the active protease.
This latter question is important to resolve since a number of clinical tests involving unprocessed or incorrectly processed PSA are either currently in use or under evaluation in an effort to improve the predictive value of the PSA test [19]. The PSA-activating protease may also play an important role in advanced prostate cancer since even poorly differentiated prostate cancer cells obtained at autopsy from castrate resistant patients maintain both production of high amounts of PSA as well as the enzymatic machinery required to process PSA to an enzymatically active form [1].
Several trypsin-like proteases, including trypsin [20], urokinase [20], and the previously mentioned kallikreins [21], have been described that could either activate pro-PSA or cleave a fluorescent substrate made from the pro-sequence. A kallikrein appears to be the most likely candidate PSA-activator since, with the exception of PSA, KLK7, and KLK9, all have trypsin-like activity and most (i.e., KLKs 1, 2, 4, 5, 6, 8, 10, 11, 14, and 15) are either expressed in the prostate or the seminal fluid [22]. KLK2 has attracted most attention for several reasons. KLK2 is 80% homologous to PSA, including in its pro-domain (VPLIQSR verses APLILSR for PSA) which it effectively removes during auto-activation [20]. KLK2 expression is elevated in prostate cancer and can be found in the blood [23].
Several reports have documented that KLK2 can activate PSA using cell-free settings that, while demonstrating the biochemical potential of the candidate protease to act on PSA, are limited in what can be interpreted biologically. Our studies were designed to characterize the processing and activation of PSA by KLK2 in vivo. These studies were performed in the mouse, which lacks both genes. In fact, only old world monkey, primate, and human genomes contain KLK2 and PSA [13]. We confirmed by co-incubation that PSA is activated by KLK2 both when PSA and KLK2-expressing clones are physically in contact, and through conditioned media. Subsequently, we co-inoculated these cells subcutaneously and demonstrated that the free-(inactive PSA) to-total (active and inactive PSA) ratio in the blood was altered, consistent with increased activation of PSA by KLK2-expressing Du145 cells. The lack of active PSA in the control blood suggests that any mouse kallikreins present in the subcutaneous compartment were unable to activate PSA.
We also generated transgenic animals expressing either PSA or KLK2 and crossed them to produce PSA/KLK2 double-transgenic animals. Previously, Wei et al. [24] generated transgenic mice expressing PSA in the prostate to evaluate mechanisms of immunomodulation. However, this group did not report on the activity of PSA in this system. Additionally, we evaluated the prostates from these animals with the PSA-selective flourogenic substrate used in this study, and found the PSA it produced to be inactive (unpublished data). In our study, PSA and KLK2 expression was directed specifically to the prostate using the (ARR)2 PB promoter. This system produced similar levels of PSA expression in the prostates of the PSA and PSA/KLK2 mice but minimal PSA activity in the absence of KLK2. In contrast, PSA activity increased fourfold in the presence of KLK2. As with the subcutaneous model, the low level of PSA activity in the PSA-only mice suggests that the mouse prostate lacks the appropriate PSA-processing enzyme.
Accumulating evidence suggests that PSA may play a role in inflammation, in cleavage of biologically relevant cytokines, and in degradation of basement membrane components [25]. We wanted to use our transgenic models to evaluate whether continued expression of active PSA over the life of the adult animal would cause morphologic changes or carcinogenesis in the prostate. The initial conclusion from these studies is that PSA or KLK2 (unpublished data) do not by themselves or combined appear to play a role in the initiation of prostate cancer. However, there are multiple complicating limitations to the mouse model. First, the levels of PSA produced by this transgenic system are up to 1,000-fold lower than the human prostate. Additionally, there are gross anatomical differences between mouse and human prostates, and the aging mouse prostate does not exhibit the same degree of inflammatory pathology as that observed for the aging human [26]. Interestingly, the PSA transgenic mice had either no detectable PSA or low levels of PSA in the serum even out to 24 months, while no detectable PSA was found in the serum of the PSA/KLK2 mice out to 10 months of age. Our findings in the transgenic mouse suggest there may be differences in PSA flux between species that might limit the utility of the mouse prostate as a model system for assessing PSA’s role in the initiation of prostate cancer.
The demonstration that PSA in either an active or inactive form does not initiate prostate cancer in mice suggests that PSA expression is not sufficient to initiate neoplastic changes. Other known oncogenes and tumor suppressors have also displayed no tumor-inducing effects in mouse transgenic systems modeling prostate cancer and need to be modulated in combination with another oncogene or tumor suppressor [27]. The next step in the evaluation of PSA’s role in prostate cancer development must be to cross these PSA/KLK2 mice with mouse models of early stage of prostate cancer, including the lo-myc [28] and skp2 [29] mouse models, which develop low-grade and high-grade prostatic intraepithelial neoplasia (PIN). These contexts may determine if PSA can drive progression from PIN to invasive prostate cancer.
REFERENCES
- 1.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (“warm“) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6(3):1038–1045. [PubMed] [Google Scholar]
- 2.Kumar A, Mikolajczyk SD, Hill TM, Millar LS, Saedi MS. Different proportions of various prostate-specific antigen (PSA) and human kallikrein 2 (hK2) forms are present in noninduced and androgen-induced LNCaP cells. Prostate. 2000;44(3):248–254. doi: 10.1002/1097-0045(20000801)44:3<248::aid-pros10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 3.Paliouras M, Diamandis EP. The kallikrein world: An update on the human tissue kallikreins. Biol Chem. 2006;387(6):643–652. doi: 10.1515/BC.2006.083. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Mikolajczyk SD, Goel AS, Millar LS, Saedi MS. Expression of pro form of prostate-specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res. 1997;57(15):3111–3114. [PubMed] [Google Scholar]
- 5.Lovgren J, Rajakoski K, Karp M, Lundwall a, Lilja H. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Commun. 1997;238(2):549–555. doi: 10.1006/bbrc.1997.7333. [DOI] [PubMed] [Google Scholar]
- 6.Takayama TK, Fujikawa K, Davie EW. Characterization of the precursor of prostate-specific antigen. Activation by trypsin and by human glandular kallikrein. J Biol Chem. 1997;272(34):21582–21588. doi: 10.1074/jbc.272.34.21582. [DOI] [PubMed] [Google Scholar]
- 7.Vaisanen V, Lovgren J, Hellman J, Piironen T, Lilja H, Pettersson K. Characterization and processing of prostate specific antigen (hK3) and human glandular kallikrein (hK2) secreted by LNCaP cells. Prostate Cancer Prostatic Dis. 1999;2(2):91–97. doi: 10.1038/sj.pcan.4500289. [DOI] [PubMed] [Google Scholar]
- 8.Denmeade SR, Sokoll LJ, Chan DW, Khan SR, Isaacs JT. Concentration of enzymatically active prostate-specific antigen (PSA) in the extracellular fluid of primary human prostate cancers and human prostate cancer xenograft models. Prostate. 2001;48(1):1–6. doi: 10.1002/pros.1075. [DOI] [PubMed] [Google Scholar]
- 9.Takayama TK, McMullen BA, Nelson PS, Matsumura M, Fujikawa K. Characterization of hK4 (prostase), a prostate-specific serine protease: Activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry. 2001;40(50):15341–15348. doi: 10.1021/bi015775e. [DOI] [PubMed] [Google Scholar]
- 10.Emami N, Diamandis EP. Human kallikrein-related peptidase 14 (KLK14) is a new activator component of the KLK proteolytic cascade. Possible function in seminal plasma and skin. J Biol Chem. 2008;283(6):3031–3041. doi: 10.1074/jbc.M707253200. [DOI] [PubMed] [Google Scholar]
- 11.Takayama TK, Carter CA, Deng T. Activation of prostate-specific antigen precursor (pro-PSA) by prostin, a novel human prostatic serine protease identified by degenerate PCR. Biochemistry. 2001;40(6):1679–1687. doi: 10.1021/bi002129r. [DOI] [PubMed] [Google Scholar]
- 12.Pampalakis G, Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim Biophys Acta. 2007;1776(1):22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Lundwall A, Clauss A, Olsson AY. Evolution of kallikrein-related peptidases in mammals and identification of a genetic locus encoding potential regulatory inhibitors. Biol Chem. 2006;387(3):243–249. doi: 10.1515/BC.2006.032. [DOI] [PubMed] [Google Scholar]
- 14.Denmeade SR, Lou W, Lovgren J, Malm J, Lilja H, Isaacs JT. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate-specific antigen. Cancer Res. 1997;57(21):4924–4930. [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SR, Denmeade SR. In vivo activity of a PSA-activated doxorubicin prodrug against PSA-producing human prostate cancer xenografts. Prostate. 2000;45(1):80–83. doi: 10.1002/1097-0045(20000915)45:1<80::aid-pros10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141(12):4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 17.Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, Denmeade SR. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99(5):376–385. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen S, Jakobsen CM, Rosen DM, Ricklis RM, Reineke U, Christensen SB, Lilja H, Denmeade SR. Screening a combinatorial peptide library to develop a human glandular kallikrein 2-activated prodrug as targeted therapy for prostate cancer. Mol Cancer Ther. 2004;3(11):1439–1450. [PubMed] [Google Scholar]
- 19.Sokoll LJ, Wang Y, Feng Z, Kagan J, Partin AW, Sanda MG, Thompson IM, Chan DW. [-2]proenzyme prostate specific antigen for prostate cancer detection: A national cancer institute early detection research network validation study. J Urol. 2008;180(2):539–543. doi: 10.1016/j.juro.2008.04.015. discussion 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denmeade SR, Lovgren J, Khan SR, Lilja H, Isaacs JT. Activation of latent protease function of pro-hK2, but not pro-PSA, involves auto processing. Prostate. 2001;48(2):122–126. doi: 10.1002/pros.1088. [DOI] [PubMed] [Google Scholar]
- 21.Yoon H, Laxmikanthan G, Lee J, Blaber SI, Rodriguez A, Kogot JM, Scarisbrick IA, Blaber M. Activation profiles and regulatory cascades of the human kallikrein-related peptidases. J Biol Chem. 2007;282(44):31852–31864. doi: 10.1074/jbc.M705190200. [DOI] [PubMed] [Google Scholar]
- 22.Veveris-Lowe TL, Kruger SJ, Walsh T, Gardiner RA, Clements JA. Seminal fluid characterization for male fertility and prostate cancer: Kallikrein-related serine proteases and whole proteome approaches. Semin Thromb Hemost. 2007;33(1):87–99. doi: 10.1055/s-2006-958467. [DOI] [PubMed] [Google Scholar]
- 23.Partin AW, Catalona WJ, Finlay JA, Darte C, Tindall DJ, Young CY, Klee GG, Chan DW, Rittenhouse HG, Wolfert RL, Woodrum DL. Use of human glandular kallikrein 2 for the detection of prostate cancer: Preliminary analysis. Urology. 1999;54(5):839–845. doi: 10.1016/s0090-4295(99)00270-8. [DOI] [PubMed] [Google Scholar]
- 24.Wei C, Willis RA, Tilton BR, Looney RJ, Lord EM, Barth RK, Frelinger JG. Tissue-specific expression of the human prostate-specific antigen gene in transgenic mice: Implications for tolerance and immunotherapy. Proc Natl Acad Sci USA. 1997;94(12):6369–6374. doi: 10.1073/pnas.94.12.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67(3):312–329. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- 26.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: Implications for prostatic carcinogenesis. Am J Pathol. 1999;155(6):1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasper S. Survey of genetically engineered mouse models for prostate cancer: Analyzing the molecular basis of prostate cancer development, progression, and metastasis. J Cell Biochem. 2005;94(2):279–297. doi: 10.1002/jcb.20339. [DOI] [PubMed] [Google Scholar]
- 28.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 29.Shim EH, Johnson L, Noh HL, Kim YJ, Sun H, Zeiss C, Zhang H. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63(7):1583–1588. [PubMed] [Google Scholar]