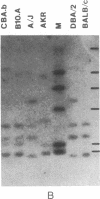
Abstract
A family of murine anti-p-azophenylarsonate (Ars) antibodies share a variable (V) region serologically defined marker, the 36-60 idiotype (Id36-60). Most mouse strains possess five genes highly homologous to the gene encoding the heavy (H) chain V region of antibodies bearing Id36-60 (VH36-60); however, only one of these genes is ever utilized by hybridomas whose antibodies bind Ars and bear Id36-60. The relevant VH genes were cloned from A/J and BALB/c mouse DNA libraries. Their DNA sequences were found to differ at only two positions. Southern blot analysis, protein sequence determination, and nucleic acid sequence determination indicate that the above hybridomas utilize the same joining (JH3), diversity (D), and VH gene segments regardless of BALB/c or A/J strain origin. Despite this virtual identity, BALB/c and A/J mouse strains express quite different serum levels of Id36-60-bearing antibodies when immunized with Ars. The basis of this regulatory process is discussed.
Full text
PDF
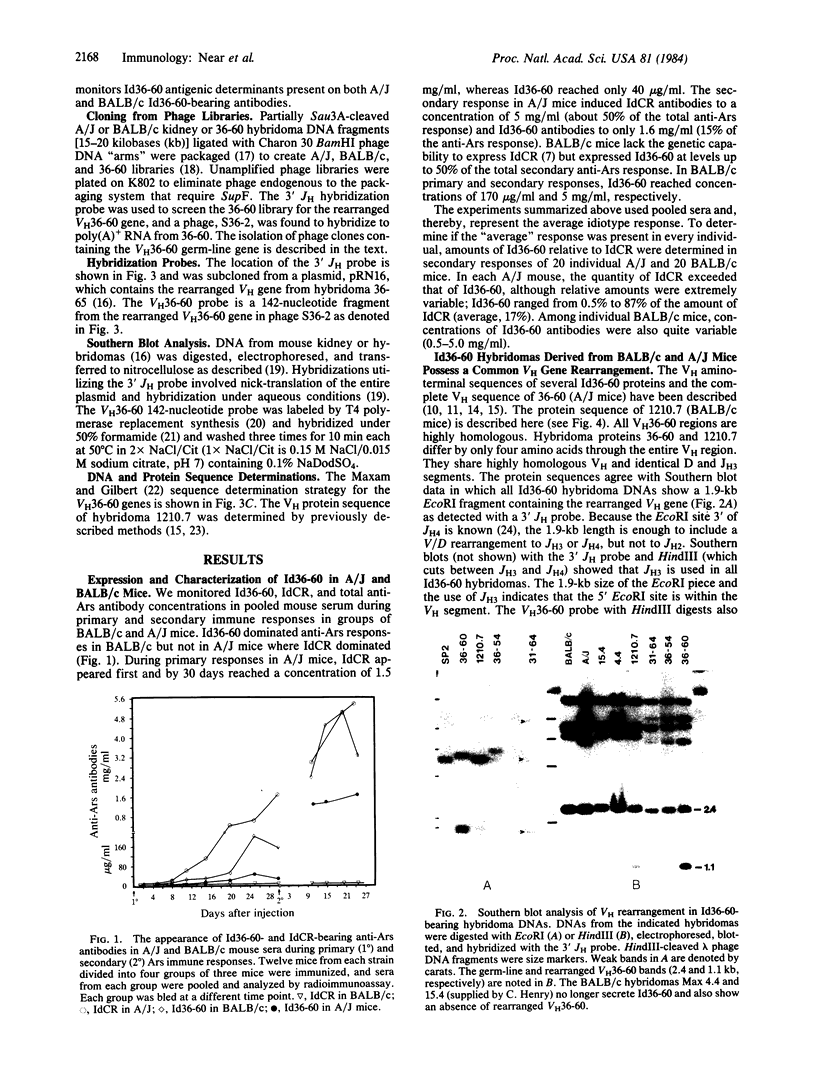



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan S. S., Ball R. K., Chang J. Y., Braun D. G. Heterogeneity of cross-reactive idiotypes. Serological and structural analysis of monoclonal anti-p-azobenzene-arsonate antibodies expressing major and minor idiotypes. Mol Immunol. 1983 Feb;20(2):203–211. doi: 10.1016/0161-5890(83)90132-3. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brown A. R., Nisonoff A. An intrastrain cross-reactive idiotype associated with anti-p-azophenylarsonate antibodies of BALB/c mice. J Immunol. 1981 Apr;126(4):1263–1267. [PubMed] [Google Scholar]
- Clarke S. H., Claflin J. L., Potter M., Rudikoff S. Polymorphism in anti-phosphocholine antibodies reflecting evolution of immunoglobulin families. J Exp Med. 1983 Jan 1;157(1):98–113. doi: 10.1084/jem.157.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. H., Claflin J. L., Rudikoff S. Polymorphism in immunoglobulin heavy chains suggesting gene conversion. Proc Natl Acad Sci U S A. 1982 May;79(10):3280–3284. doi: 10.1073/pnas.79.10.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science. 1972 Jun 2;176(4038):1027–1029. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Juszczak E. C., Margolies M. N. Amino acid sequence of the heavy chain variable region from the A/J mouse anti-arsonate monoclonal antibody 36-60 bearing a minor idiotype. Biochemistry. 1983 Aug 30;22(18):4291–4296. doi: 10.1021/bi00287a020. [DOI] [PubMed] [Google Scholar]
- Krawinkel U., Zoebelein G., Brüggemann M., Radbruch A., Rajewsky K. Recombination between antibody heavy chain variable-region genes: evidence for gene conversion. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4997–5001. doi: 10.1073/pnas.80.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin J. A., Gray A., Nisonoff A., Klinman N. R., Gottlieb P. D. Segregation at a locus determining an immunoglobulin genetic marker for the light chain variable region affects inheritance of expression of an idiotype. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4600–4604. doi: 10.1073/pnas.74.10.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolies M. N., Juszczak E. C., Near R., Marshak-Rothstein A., Rothstein T. L., Sato V. L., Siekevitz M., Smith J. A., Wysocki L. J., Gefter M. L. Structural correlates of idiotypy in the arsonate system. Ann N Y Acad Sci. 1983;418:48–64. doi: 10.1111/j.1749-6632.1983.tb18054.x. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Marshak-Rothstein A., Gefter M. L. Structural diversity among anti-p-azophenylarsonate monoclonal antibodies from A/J mice; comparison of Id- and Id+ sequences. Mol Immunol. 1981 Dec;18(12):1065–1077. doi: 10.1016/0161-5890(81)90022-5. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Benedetto J. D., Kirsch R. L., Gefter M. L. Unique determinants associated with hybridoma proteins expressing a cross-reactive idiotype: frequency among individual immune sera. J Immunol. 1980 Nov;125(5):1987–1992. [PubMed] [Google Scholar]
- Marshak-Rothstein A., Margolies M. N., Benedetto J. D., Gefter M. L. Two structurally distinct and independently regulated idiotypic families associated with the A/J response to azophenylarsonate. Eur J Immunol. 1981 Jul;11(7):565–572. doi: 10.1002/eji.1830110709. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Siekevitz M., Margolies M. N., Mudgett-Hunter M., Gefter M. L. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Milner E. C., Capra J. D. VH families in the antibody response to p-azophenylarsonate: correlation between serology and amino acid sequence. J Immunol. 1982 Jul;129(1):193–199. [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Gene conversion and polymorphism: generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell. 1983 Feb;32(2):515–523. doi: 10.1016/0092-8674(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Owen F. L., Ju S. T., Nisonoff A. Binding to idiotypic determinants of large proportions of thymus-derived lymphocytes in idiotypically suppressed mice. Proc Natl Acad Sci U S A. 1977 May;74(5):2084–2088. doi: 10.1073/pnas.74.5.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak L. L., Nisonoff A. Distribution of a cross-reactive idiotypic specificity in inbred strains of mice. J Exp Med. 1973 Apr 1;137(4):855–869. doi: 10.1084/jem.137.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell J. D., Gearhart P. J., Karush F. Restriction in IgM expression. IV. Affinity analysis of monoclonal anti-phosphorylcholine antibodies. J Immunol. 1983 Jan;130(1):313–316. [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Giusti A. M., Cook W. D., Scharff M. D. Single amino acid substitution altering antigen-binding specificity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1979–1983. doi: 10.1073/pnas.79.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Gefter M. L., Brodeur P., Riblet R., Marshak-Rothstein A. The genetic basis of antibody production: the dominant anti-arsonate idiotype response of the strain A mouse. Eur J Immunol. 1982 Dec;12(12):1023–1032. doi: 10.1002/eji.1830121208. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Sigal N. H. Regulation of azophenylarsonate-specific repertoire expression. 1. Frequency of cross-reactive idiotype-positive B cells in A/J and BALB/c mice. J Exp Med. 1982 Nov 1;156(5):1352–1365. doi: 10.1084/jem.156.5.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Miller J., Storb U. Rearrangement of immunoglobulin genes. Biochemistry. 1979 Oct 30;18(22):5013–5021. doi: 10.1021/bi00589a032. [DOI] [PubMed] [Google Scholar]
- Woodland R., Cantor H. Idiotype-specific T helper cells are required to induce idiotype-positive B memory cells to secrete antibody. Eur J Immunol. 1978 Aug;8(8):600–606. doi: 10.1002/eji.1830080812. [DOI] [PubMed] [Google Scholar]