Abstract
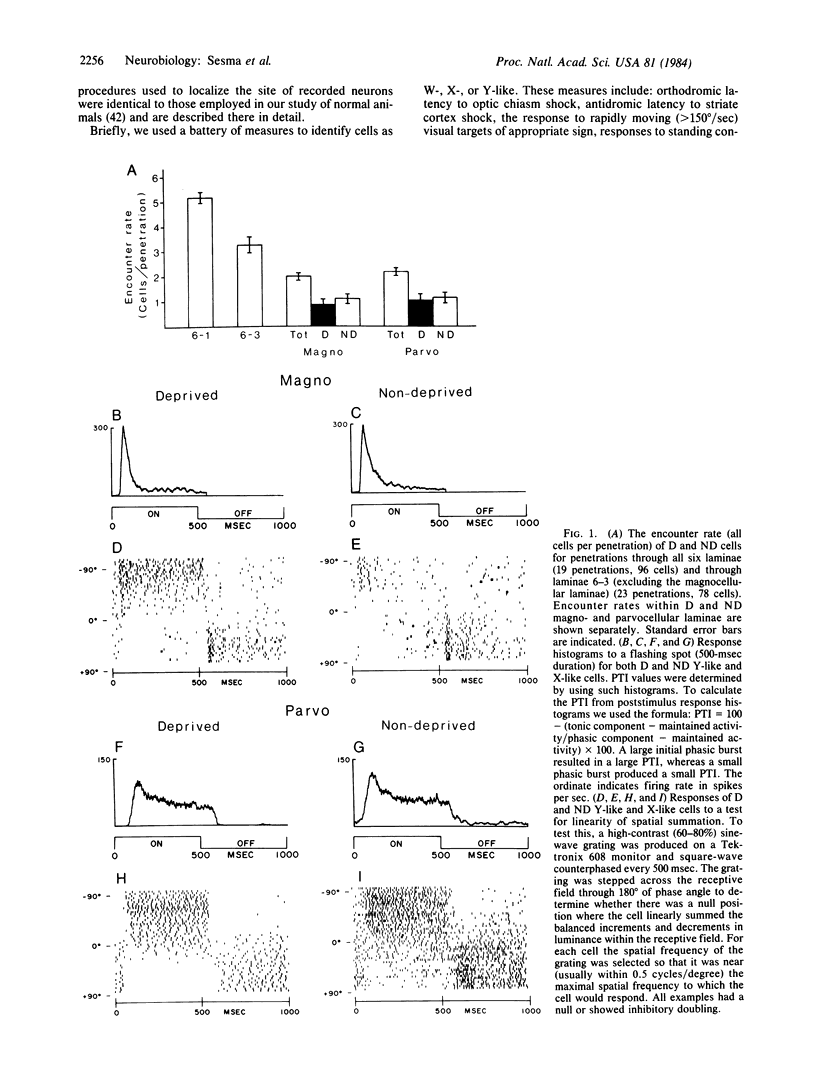
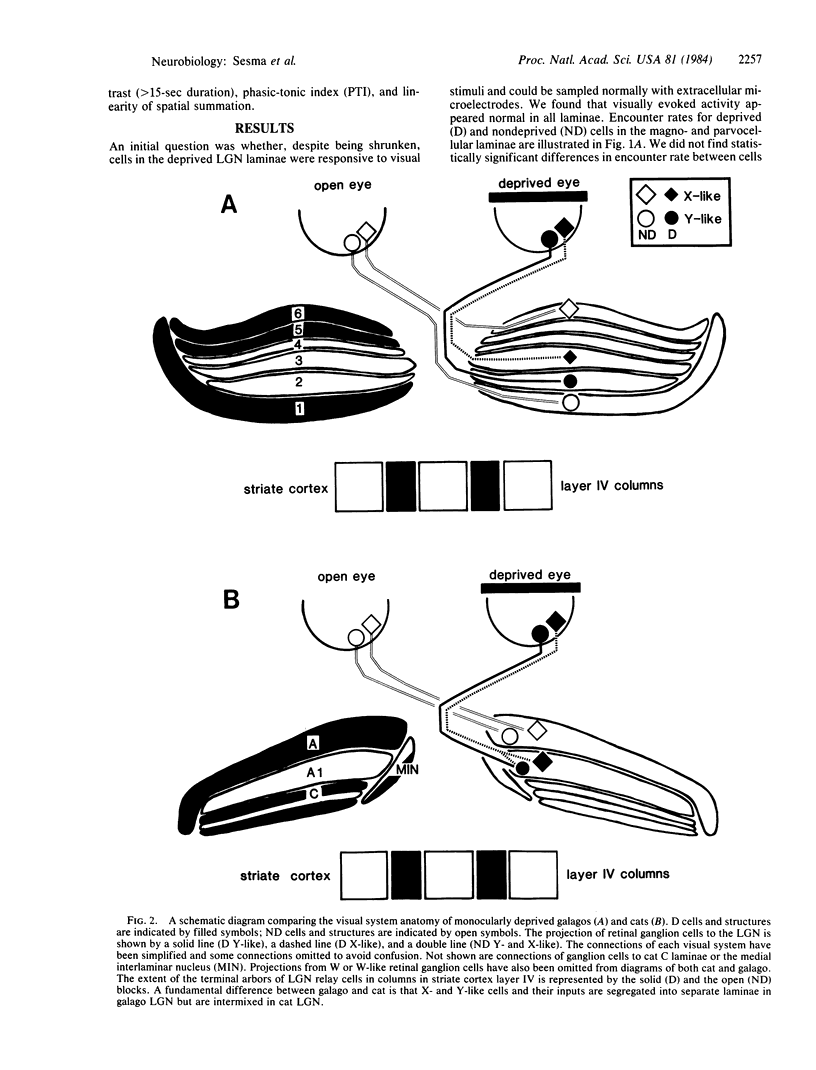
In many mammalian species, rearing with one eyelid closed produces a loss of vision in the deprived eye and a change in cell size in the lateral geniculate nucleus (LGN). In cats, the reduction in the size of deprived LGN cells has been correlated with a loss of one functional class of cells, Y cells. In primates, such as galago, LGN cells also exhibit marked changes in size with deprivation. In the present study we recorded from single cells in the LGN of monocularly deprived galagos to determine if such changes in cell size would be accompanied by changes in physiological properties. The results revealed no alterations in the distribution or functional properties of any cell class. The differences in the effects of monocular deprivation on the function of LGN cells in cats and primates are most easily explained by a fundamental difference in visual system anatomy. In cats, different classes of retinal afferents (X vs. Y) are in a position to compete for postsynaptic LGN neurons: in primates, segregation of cell classes into different layers may preclude such developmental interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Garey L. J., Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978 Oct;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F. Development of the neural basis of visual acuity in monkeys: speculation on the origin of deprivation amblyopia. Trans Ophthalmol Soc U K. 1979;99(3):363–368. [PubMed] [Google Scholar]
- Blakemore C., Vital-Durand F., Garey L. J. Recovery from monocular deprivation in the monkey. I. Reversal of physiological effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):399–423. doi: 10.1098/rspb.1981.0072. [DOI] [PubMed] [Google Scholar]
- Casagrande V. A., Guillery R. W., Harting J. K. Differential effects of monocular deprivation seen in different layers of the lateral geniculate nucleus. J Comp Neurol. 1978 Jun 1;179(3):469–485. doi: 10.1002/cne.901790302. [DOI] [PubMed] [Google Scholar]
- Casagrande V. A., Joseph R. Morphological effects of monocular deprivation and recovery on the dorsal lateral geniculate nucleus in galago. J Comp Neurol. 1980 Nov 15;194(2):413–426. doi: 10.1002/cne.901940208. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Hawken M. J. Spatial and temporal properties of cat geniculate neurones after prolonged deprivation. J Physiol. 1981 May;314:107–120. doi: 10.1113/jphysiol.1981.sp013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. J., Stanford L. R., Sherman S. M. Effects of monocular deprivation on the structure-function relationship of individual neurons in the cat's lateral geniculate nucleus. J Neurosci. 1982 Mar;2(3):321–330. doi: 10.1523/JNEUROSCI.02-03-00321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. The effect of lid suture upon the growth of cells in the dorsal lateral geniculate nucleus of kittens. J Comp Neurol. 1973 Apr 15;148(4):417–422. doi: 10.1002/cne.901480402. [DOI] [PubMed] [Google Scholar]
- Headon M. P., Powell T. P. Cellular changes in the lateral geniculate nucleus of infant monkeys after suture of the eyelids. J Anat. 1973 Oct;116(Pt 1):135–145. [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A., Boles J., McLean E. B. Visual acuity and behavior of monocularly deprived monkeys after retinal lesions. Invest Ophthalmol Vis Sci. 1977 May;16(5):469–473. [PubMed] [Google Scholar]
- Hickey T. L., Spear P. D., Kratz K. E. Quantitative studies of cell size in the cat's dorsal lateral geniculate nucleus following visual deprivation. J Comp Neurol. 1977 Mar 15;172(2):265–281. doi: 10.1002/cne.901720206. [DOI] [PubMed] [Google Scholar]
- Hof-Van Duin J. V. Early and permanent effects of monocular deprivation on pattern discrimination and visuomotor behavior in cats. Brain Res. 1976 Jul 30;111(2):261–276. doi: 10.1016/0006-8993(76)90771-x. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb Symp Quant Biol. 1976;40:581–589. doi: 10.1101/sqb.1976.040.01.054. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Joseph R., Casagrande V. A. Visual deficits and recovery following monocular lid closure in a prosimian primate. Behav Brain Res. 1980 Apr;1(2):165–186. doi: 10.1016/s0166-4328(80)80055-6. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Huerta M. F., Weber J. T., Harting J. K. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J Comp Neurol. 1978 Dec 1;182(3):517–553. doi: 10.1002/cne.901820308. [DOI] [PubMed] [Google Scholar]
- Kalil R. Dark rearing in the cat: effects on visuomotor behavior and cell growth in the dorsal lateral geniculate nucleus. J Comp Neurol. 1978 Apr 1;178(3):451–467. doi: 10.1002/cne.901780304. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Sherman S. M., Kalil R. Lateral geniculate nucleus in dark-reared cats: loss of Y cells without changes in cell size. Science. 1979 Mar 30;203(4387):1353–1355. doi: 10.1126/science.424758. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Webb S. V., Sherman S. M. Effects of early monocular lid suture upon neurons in the cat's medial interlaminar nucleus. J Comp Neurol. 1978 Oct 1;181(3):615–625. doi: 10.1002/cne.901810309. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S., Kratz K. E., Mangel S. C., Sherman S. M. Effects of early monocular lid suture on spatial and temporal sensitivity of neurons in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1980 Feb;43(2):542–556. doi: 10.1152/jn.1980.43.2.542. [DOI] [PubMed] [Google Scholar]
- Mangel S. C., Wilson J. R., Sherman S. M. Development of neuronal response properties in the cat dorsal lateral geniculate nucleus during monocular deprivation. J Neurophysiol. 1983 Jul;50(1):240–264. doi: 10.1152/jn.1983.50.1.240. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Van Sluyters R. C. Visual neural development. Annu Rev Psychol. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Mower G. D., Christen W. G. Effects of early monocular deprivation on the acuity of lateral geniculate neurons in the cat. Brain Res. 1982 Mar;255(3):475–480. doi: 10.1016/0165-3806(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Norton T. T., Casagrande V. A. Laminar organization of receptive-field properties in lateral geniculate nucleus of bush baby (Galago crassicaudatus). J Neurophysiol. 1982 Apr;47(4):715–741. doi: 10.1152/jn.1982.47.4.715. [DOI] [PubMed] [Google Scholar]
- Norton T. T., Casagrande V. A., Sherman S. M. Loss of Y-cells in the lateral geniculate nucleus of monocularly deprived tree shrews. Science. 1977 Aug 19;197(4305):784–786. doi: 10.1126/science.887922. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. M., Guillery R. W., Kaas J. H., Sanderson K. J. Behavioral, electrophysiological and morphological studies of binocular competition in the development of the geniculo-cortical pathways of cats. J Comp Neurol. 1974 Nov 1;158(1):1–18. doi: 10.1002/cne.901580102. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Hoffmann K. P., Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 1972 Jul;35(4):532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Wilson J. R. Behavioral and morphological evidence for binocular competition in the postnatal development of the dog's visual system. J Comp Neurol. 1975 May 15;161(2):183–195. doi: 10.1002/cne.901610204. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Wilson J. R., Kaas J. H., Webb S. V. X- and Y-cells in the dorsal lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). Science. 1976 Apr 30;192(4238):475–477. doi: 10.1126/science.816006. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B., Leventhal A. Hierarchical and parallel mechanisms in the organization of visual cortex. Brain Res. 1979 Dec;180(3):345–394. doi: 10.1016/0165-0173(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Sur M., Humphrey A. L., Sherman S. M. Monocular deprivation affects X- and Y-cell retinogeniculate terminations in cats. Nature. 1982 Nov 11;300(5888):183–185. doi: 10.1038/300183a0. [DOI] [PubMed] [Google Scholar]
- Swindale N. V., Vital-Durand F., Blakemore C. Recovery from monocular deprivation in the monkey. III. Reversal of anatomical effects in the visual cortex. Proc R Soc Lond B Biol Sci. 1981 Nov 24;213(1193):435–450. doi: 10.1098/rspb.1981.0074. [DOI] [PubMed] [Google Scholar]
- Von Noorden G. K., Crawford M. L., Middle-Ditch P. R. The effects of monocular visual deprivation: disuse or binocular interaction? Brain Res. 1976 Jul 30;111(2):277–285. doi: 10.1016/0006-8993(76)90772-1. [DOI] [PubMed] [Google Scholar]
- Von Noorden G. K. Factors involved in the production of amblyopia. Br J Ophthalmol. 1974 Mar;58(3):158–164. doi: 10.1136/bjo.58.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974 Dec 1;158(3):307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Webb S. V., Sherman S. M. Conditions for dominance of one eye during competitive development of central connections in visually deprived cats. Brain Res. 1977 Nov 11;136(2):277–287. doi: 10.1016/0006-8993(77)90803-4. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K. Experimental amblyopia in monkeys. Further behavioral observations and clinical correlations. Invest Ophthalmol. 1973 Oct;12(10):721–726. [PubMed] [Google Scholar]
- von Noorden G. K. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol. 1973 Oct;12(10):727–738. [PubMed] [Google Scholar]
- von Noorden G. K., Middleditch P. R. Histology of the monkey lateral geniculate nucleus after unilateral lid closure and experimental strabismus: further observations. Invest Ophthalmol. 1975 Sep;14(9):674–683. [PubMed] [Google Scholar]


