Abstract
An appropriate response and adaptation to hyperosmolarity, i.e., an external osmolarity that is higher than the physiological range, can be a matter of life or death for all cells. It is especially important for free-living organisms such as the yeast Saccharomyces cerevisiae. When exposed to hyperosmotic stress, the yeast initiates a complex adaptive program that includes temporary arrest of cell-cycle progression, adjustment of transcription and translation patterns, and the synthesis and retention of the compatible osmolyte glycerol. These adaptive responses are mostly governed by the high osmolarity glycerol (HOG) pathway, which is composed of membrane-associated osmosensors, an intracellular signaling pathway whose core is the Hog1 MAP kinase (MAPK) cascade, and cytoplasmic and nuclear effector functions. The entire pathway is conserved in diverse fungal species, while the Hog1 MAPK cascade is conserved even in higher eukaryotes including humans. This conservation is illustrated by the fact that the mammalian stress-responsive p38 MAPK can rescue the osmosensitivity of hog1Δ mutations in response to hyperosmotic challenge. As the HOG pathway is one of the best-understood eukaryotic signal transduction pathways, it is useful not only as a model for analysis of osmostress responses, but also as a model for mathematical analysis of signal transduction pathways. In this review, we have summarized the current understanding of both the upstream signaling mechanism and the downstream adaptive responses to hyperosmotic stress in yeast.
SACCHAROMYCES (literally, sugar yeast) thrive, in their natural habitat, on decomposing fruits, including grape, where sugar (such as glucose, fructose, and sucrose) is abundant. As the fruits dry, the sugar concentration may approach its saturation point. This high sugar concentration poses a dilemma to the yeast, as the abundant food also brings unfavorable osmotic conditions that are a potential threat to their survival. Increased external osmolarity induces water efflux, an increased concentration of cytosolic ions (especially Na+), and cell shrinkage, which are all detrimental to cell growth [for general biological effects of osmostress, see Wood (1999, 2011)]. Amazingly, yeast can grow and vigorously ferment in media containing as much as 40% (2.2 M) glucose (Watanabe et al. 2010), which is obviously a highly dangerous osmotic condition.
Therefore, to cope with such an increased external osmolarity, yeast initiates a complex adaptive program that includes temporary arrest of cell-cycle progression, adjustment of transcription and translation patterns, and the synthesis and retention of the compatible osmolyte glycerol (Figure 1). These adaptive responses are mostly governed by the high osmolarity glycerol (HOG) signaling pathway, whose core is the Hog1 MAP kinase (MAPK) cascade. In this review, we have summarized the current, often fragmentary, understanding of both the upstream signaling mechanism of osmostress and the downstream adaptive responses. Because the HOG pathway is highly conserved across fungal species, elucidation of the signaling and effector mechanisms in Saccharomyces cerevisiae will be highly relevant to the studies of other yeasts and fungi (Krantz et al. 2006a,b). We endeavored to be as comprehensive as possible, but due to space limitations, many interesting subjects had to be left out. Readers who are interested in various aspects of yeast osmostress responses are encouraged to consult a number of excellent review articles (Gustin et al. 1998; Sprague 1998; Chellappan 2001; Hohmann 2002a,b, 2009; O’Rourke et al. 2002; Saito and Tatebayashi 2004; Schwartz and Madhani 2004; Sheikh-Hamad and Gustin 2004; Chen and Thorner 2007; Hohmann et al. 2007; de Nadal and Posas 2010).
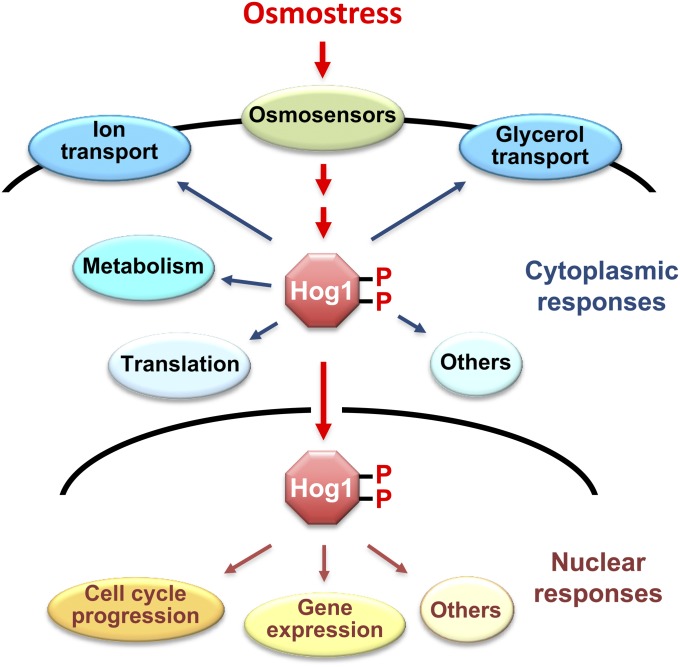
Figure 1 .
Osmo-adaptive responses in yeast. In response to an increase in extracellular osmolarity, the Hog1 MAPK is activated, which leads to the induction of cytoplasmic and nuclear adaptive responses. Cytoplasmic responses include the control of ionic fluxes and glycerol transport, metabolic enzymes, and protein translation. Nuclear responses include the modulation of cell-cycle progression and the control of gene expression.
Upstream Signaling Mechanisms
Overview of the HOG pathway
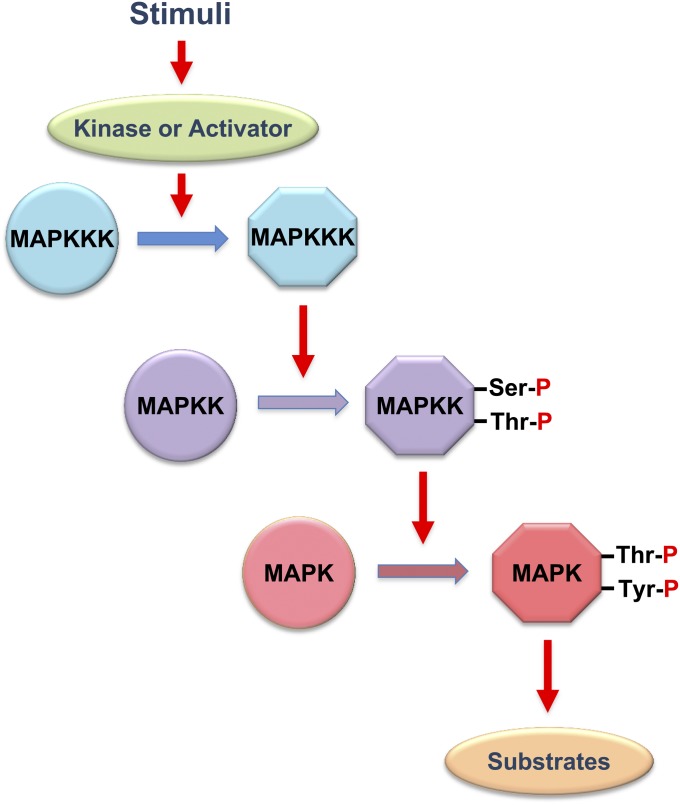
The central core of the HOG pathway is the Hog1 MAPK cascade. MAPK cascades are evolutionarily conserved signaling units that are utilized in many intracellular signal transduction pathways in diverse eukaryotic organisms, including fungi and yeast (Chen et al. 2001). Each MAPK cascade is composed of three sequentially activating kinases (Figure 2). A MAPK is activated by a MAPK kinase (MAPKK) by dual phosphorylation of the conserved Thr and Tyr residues in the TXY motif within the activation loop. A MAPKK is similarly activated by a MAPKK kinase (MAPKKK) by phosphorylation of the Ser/Thr residues in its activation loop. The first kinase of the cascade, MAPKKK, is activated either by phosphorylation by an upstream kinase, sometimes called MAPKKKK, or by binding of an activator protein, depending on the pathway. Each MAPK module is activated by specific types of stimuli and induces specific adaptive responses.
Figure 2 .
A schematic diagram of the MAP kinase module. Circles and hexagons represent, respectively, inactive and active forms of kinases. MAPK, MAP kinase; MAPKK, MAPK kinase; MAPKKK, MAPKK kinase.
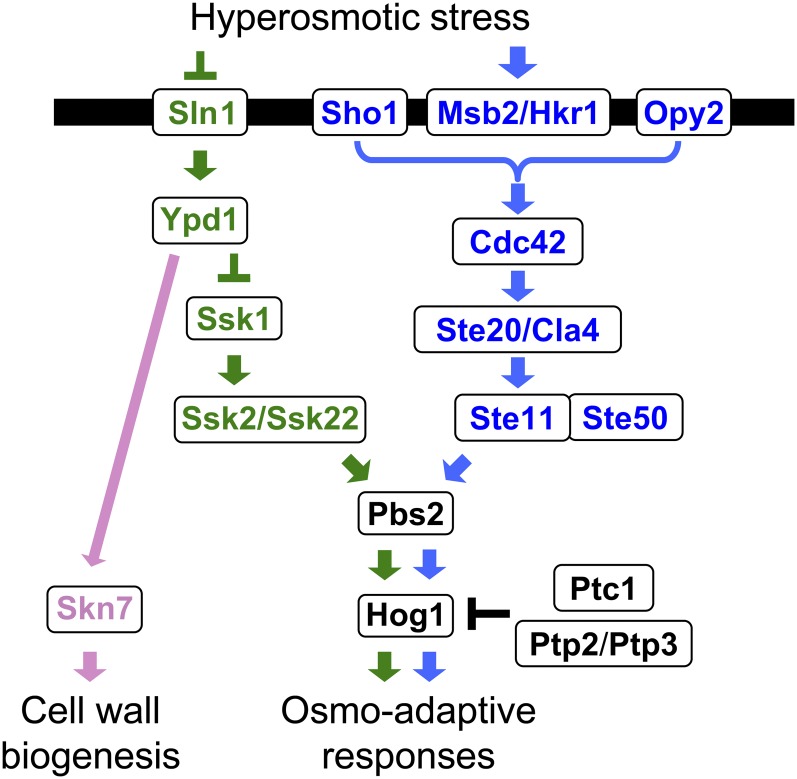
The upstream part of the HOG pathway comprises the functionally redundant, but mechanistically distinct, Sln1 and Sho1 branches (Figure 3). A signal emanating from either branch converges on a common MAPKK, Pbs2, which is the specific activator of the Hog1 MAPK (Brewster et al. 1993; Maeda et al. 1994). The Sln1 branch activates the redundant Ssk2 and Ssk22 MAPKKKs, which then activate Pbs2 (Maeda et al. 1995). The Sho1 branch activates the Ste11 MAPKKK, which also activates Pbs2 (Posas and Saito 1997). Thus, a mutant that lacks both the SSK2 and SSK22 genes (an ssk2Δ ssk22Δ mutant) is totally dependent on the Sho1 branch for activation of the Hog1 MAPK, whereas a mutant that lacks STE11 is dependent on the Sln1 branch. Once activated, a substantial fraction of the Hog1 MAPK is transported into the nucleus where it regulates transcription and the cell cycle, although there are also Hog1 targets in the cytoplasm. As adaptation proceeds, and osmotic balance is re-established, Hog1 activity goes down to near basal levels, and Hog1 is exported back to the cytoplasm. Thus, there are mechanisms that control Hog1 nuclear import/export, as well as downregulation of Hog1 activity.
Figure 3 .
A schematic diagram of the yeast HOG pathway. The protein names separated by a thrash (/) are functionally redundant. Proteins that are specific to the Sln1 branch are colored green, those that are specific to the Sho1 branch are colored blue, and those that are common are colored black. The black horizontal bar represents the plasma membrane. Arrows indicate activation, whereas the T-shaped bars represent inhibition.
There are several other signal pathways that utilize a MAPK cascade in yeast, which are involved in the mating response, filamentous and invasive growth (FIG), and regulation of cell-wall biogenesis. Surprisingly, three of these pathways (HOG, mating, and FIG) share many of the same signaling elements, including the Ste11 MAPKKK. Thus, it is important to prevent signal leakage from one pathway into another pathway. This aim seems to be attained by insulation and exquisite network of reciprocal cross-regulation among the signaling pathways.
Sln1 branch of the HOG pathway
Two-component signal transduction system:
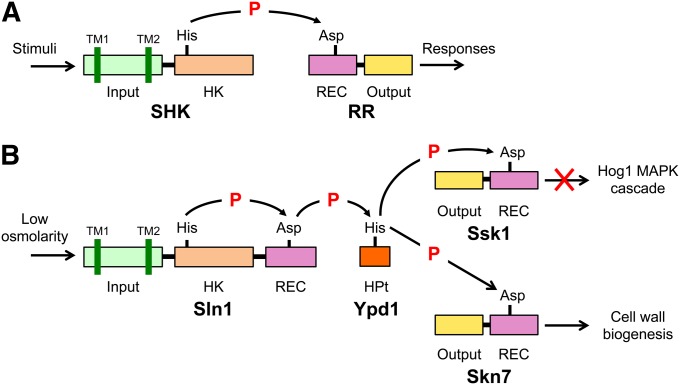
The Sln1 branch of the HOG pathway is a variation of the so-called two-component system. Two-component systems are ubiquitous in prokaryotes, plants, and fungi (for comprehensive reviews, see Stock et al. 2000; Gao and Stock 2009; Casino et al. 2010; Schaller et al. 2011). As the name implies, the prototypical two-component system is composed of two proteins (Figure 4A): the first is a sensor histidine kinase (SHK) that contains an input (or sensor) domain, a HK catalytic domain, and a histidine auto-phosphorylation site, and the second is a response regulator (RR) that contains an output (or effector) domain and a receiver (REC) domain. When the input domain senses a relevant stimulus, the HK is activated (or inactivated), and a histidine residue located near the HK domain is phosphorylated (or dephosphorylated). This phosphoryl group is then transferred to the acceptor aspartate residue in the REC domain of a cognate RR. This phosphotransfer reaction is termed the His-Asp phosphorelay. Because both histidine phosphate and aspartate phosphate are energetically activated, they are often symbolized as His∼P and Asp∼P. In bacteria, numerous simple two-component systems exist that are composed of an SHK and a cognate RR. However, there are also more complex variations of this theme, where the basic His-Asp phosphorelay reaction is repeated twice so that a phosphoryl group is transferred sequentially through a His-Asp-His-Asp multistep phosphorelay (Figure 4B). In a complex two-component system, a phosphoryl group is initially transferred from a HK domain to a cognate REC domain as in the simple systems. This phosphoryl group, however, is then transferred to an intermediate phospho-carrier termed histidine-containing phospho-transfer (HPt) protein, which catalyzes specific phospho-transfer reactions between two REC domains. The phosphoryl group is then transferred from HPt to a second REC domain. The Sln1 branch of the yeast HOG pathway is an example of complex two-component systems (Posas et al. 1996; Saito 2001). In the budding yeast, there are three REC proteins (Sln1, Ssk1, and Skn7), but only one SHK (Sln1) and one HPt (Ypd1). In fact, Sln1 governs two distinct signaling pathways: the Sln1-Ypd1-Ssk1 multistep phosphorelay, which regulates hyper-osmolarity responses, and the Sln1-Ypd1-Skn7 multistep phosphorelay, which makes a contribution to hypo-osmolarity responses.
Figure 4 .
Schematic diagram of two-component signaling systems. (A) The prototypical two-component system that is characterized by the conserved phosphotransfer reaction between a histidine residue and an aspartate residue. (B) The Sln1-Ypd1-Ssk1 multistep phosphorelay. SHK, sensor histidine kinase; RR, response regulator; HK histidine kinase domain; REC, receiver domain; HPt, histidine-containing phospho-transfer protein; TM, transmembrane segment; P, phosphoryl group.
Sln1-Ypd1-Ssk1 multistep phosphorelay:
The N-terminal half of Sln1 is the sensor domain that is composed of an extracellular domain (ECD) flanked by two transmembrane segments, TM1 and TM2 (Ota and Varshavsky 1993; Maeda et al. 1994). The C-terminal half is composed of a HK domain and a REC domain; hence Sln1 is termed a “hybrid histidine kinase.” When activated, the Sln1 HK auto-phosphorylates His-576 near the HK domain, using ATP as a phospho-donor (Posas et al. 1996). This phosphoryl group is then transferred to Asp-1144 in the Sln1 REC domain. It is likely that the HK catalytic site of one molecule phosphorylates the His phosphorylation site in another molecule in an Sln1 dimer. The phosphate is then transferred to His-64 of Ypd1, an HPt protein. The phosphoryl group on Ypd1 is finally transferred to Asp-554 in the REC domain of Ssk1.
Regulation of Sln1 HK activity:
Genetic analyses of various mutants in the Sln1 pathway suggest that the Sln1 HK domain is catalytically active under normal osmotic conditions, whereas it is inactivated when the environmental osmolarity is increased (Maeda et al. 1994; Fassler and West 2010). In vitro reconstitution of the Sln1-Ypd1-Ssk1 multistep phosphorelay reactions supports the same conclusion (Posas et al. 1996). As expected, the ECD and its flanking transmembrane (TM) domains are important for regulation of the HK activity. For example, deletion of TM1 constitutively activates, whereas removal of both TM1 and ECD inactivates, Sln1 HK (Ostrander and Gorman 1999). In vivo, Sln1 seems to respond to changes in turgor pressure (the pressure exerted by water inside the cell against the cell wall). When yeast is exposed to high external osmolarity, turgor pressure decreases as the cytoplasm shrinks. An earlier study suggested that turgor change rather than water loss activates the HOG pathway (Tamás et al. 2000), which was later supported by biophysical analyses (Schaber et al. 2010). Consistent with these findings, Sln1 HK activity is inhibited when turgor is reduced by the antifungal antibiotic nystatin or by enzymatic removal of the cell wall (Reiser et al. 2003). Conversely, Sln1 HK activity is enhanced by increased turgor pressure caused by raised intracellular glycerol concentration (Tao et al. 1999). In a more recent study, it was found that the presence of the abundant GPI-anchored cell-wall mannoprotein Ccw12 has a role in Sln1 HK activation (Shankarnarayan et al. 2008). These results suggest that Sln1 responds to osmolarity-induced changes in the cell wall. On the other hand, it was also found that the Sln1 branch of the HOG pathway is activated when membrane fluidity is reduced by a rapid downshift in temperature to <10° or by dimethyl sulfoxide treatment (Hayashi and Maeda 2006; Panadero et al. 2006). Hypoxia also activates the Sln1 branch, perhaps by an altered membrane fluidity caused by depletion of heme and ergosterol (Hickman et al. 2011). These results suggest that Sln1 might respond to changes in the plasma membrane. Cold activation of the HOG pathway might be physiologically important because Hog1-dependent accumulation of glycerol would protect yeast from freezing. In any case, it is clear that further studies are needed to establish the biophysical nature of the stimuli that control Sln1 activity.
HPt protein Ypd1:
Ypd1 is a small protein of 167 aa and is composed of a four-helix bundle with the phospho-accepting histidine (His-64) in the middle of the third helix (Song et al. 1999; Xu and West 1999). Ypd1 interacts with three different REC domains, one each in Sln1, Ssk1, and Skn7. A systematic Ala-scanning mutagenesis of Ypd1 coupled to two-hybrid interaction analyses indicated that the REC domains of Sln1, Ssk1, and Skn7 interact with Ypd1 at overlapping binding sites (Porter et al. 2003; Porter and West 2005). The α1 helix of the Ssk1 REC domain was identified as the interaction site with Ypd1 by isolation of Ssk1 mutants that cannot interact with Ypd1 (Horie et al. 2008). The structure of a complex between Ypd1 and the REC domain of Sln1 is consistent with these mutational studies (Xu et al. 2003; Zhao et al. 2008).
Phosphotransfer reactions involving wild-type Ypd1 are very rapid, reaching steady-state levels in <5 sec in vitro (Janiak-Spens and West 2000). Thus, detailed kinetic analyses are possible only by using a rapid quench flow apparatus (Kaserer et al. 2010). Perhaps the most important finding is that phosphotransfer from Ypd1∼P to Ssk1 is both very rapid (160 sec−1) and irreversible, whereas that from Ypd1∼P to Skn7 is slower (1.4 sec−1) and readily reversible (Janiak-Spens et al. 2005). These and other kinetic properties of Ypd1 are consistent with the notion that Ssk1 is constitutively phosphorylated under normal osmotic conditions.
Activation of the Ssk2/Ssk22 MAPKKKs by Ssk1:
Ssk1 activates a pair of homologous, and functionally redundant, MAPKKKs termed Ssk2 and Ssk22 (Maeda et al. 1995). Like many other members of the MAPKKK family, the kinase catalytic domain of Ssk2/Ssk22 is near the C-terminal end, and there is an auto-inhibitory domain (AID) in the N-terminal region. Ssk1 binds to the N-terminal region of Ssk2/Ssk22, and, perhaps by conformational change, relieves the catalytic domain from inhibition by the AID (Posas and Saito 1998). Since the Sln1 HK is active under normal osmotic conditions, Ssk1 is constitutively phosphorylated by Ypd1∼P. However, under hyperosmotic conditions, unphosphorylated Ssk1-OH will accumulate, and it binds and activates Ssk2/Ssk22. Consistent with this notion, expression of unphosphorylatable Ssk1 mutants such as Ssk1-D544S or Ssk1 mutants that cannot interact with Ypd1 (and thus cannot accept phosphate from Ypd1∼P), such as Ssk1-I514T, hyperactivate the Hog1 MAPK cascade (Horie et al. 2008).
Asp∼P is chemically unstable and is spontaneously hydrolyzed. Indeed, the half-life of purified Ssk1∼P is only ∼13 min in vitro (Janiak-Spens et al. 2000). If it is similarly unstable in cells, then it is unlikely that all of the Ssk1 is stably converted to Ssk1∼P, and therefore there is a possibility that persistent Ssk1-OH would activate the Hog1 MAPK cascade in the absence of any osmotic stimulation. However, several mechanisms exist that prevent erroneous activation of the Hog1 MAPK cascade. First, the half-life of Ssk1∼P dramatically increases to 40 hr when Ypd1 is included in the incubation reaction in vitro (Janiak-Spens et al. 1999). It was proposed that Ypd1 forms a stable complex with Ssk1∼P and sterically shields the phosphorylated Asp residue from hydrolysis (Janiak-Spens et al. 2000). Such enhanced stability of Ssk1∼P would maintain the levels of Ssk1-OH in unstimulated cells at a level low enough that inadvertent activation of the Hog1 MAPK cascade would be prevented. Second, any residual Ssk1-OH that may still exist would not contribute significantly to Ssk2/Ssk22 activation because only a doubly dephosphorylated Ssk1 dimer, (Ssk1-OH)2, can activate Ssk2 and Ssk22 (Horie et al. 2008). For example, when 1% of Ssk1 is dephosphorylated, only 0.01% of Ssk1 dimer is doubly dephosphorylated. Third, Ssk1-OH is degraded by a ubiquitin-proteasome-dependent mechanism, which may serve as an additional safeguard against spontaneous activation of Ssk2/Ssk22 in the absence of osmostress (Sato et al. 2003). Finally, it should be noted that there is in fact a low basal signaling in the Sln1 pathway in the absence of any external stimulation, which may allow more rapid response upon osmostress (Macia et al. 2009).
Although stable Ssk1∼P is required to prevent spontaneous Hog1 activation under nonstimulated conditions, it causes another difficulty under high-osmolarity conditions. When yeast is exposed to hyper-osmolarity, activation of the Hog1 MAP kinase cascade occurs within minutes, which requires a much faster dephosphorylation of Ssk1∼P than the observed half-life of 40 hr in vitro in the presence of Ypd1. Higher osmolyte concentrations decrease the Ssk1∼P half-life by two-fold in in vitro reactions, but this modest effect alone would not be sufficient to account for the rapid in vivo activation of the MAPK cascade (Kaserer et al. 2009). Therefore, the dephosphorylation of Ssk1∼P might be accelerated under stress conditions in vivo, perhaps by an as-yet-unidentified phosphatase.
The actin cytoskeleton is important for the survival of yeast under osmostress, as many mutations in actin cause osmosensitivity (Wertman et al. 1992). Hyperosmotic stress causes a rapid disassembly of actin cables, followed by depolarization of actin patches leading to a cell-cycle delay (Chowdhury et al. 1992). The reassembly of the actin cytoskeleton occurs only after osmotic balance is re-established (Brewster and Gustin 1994). During osmostress, Ssk2 concentrates in the neck of budding cells and forms a complex with actin, and following reestablishment of osmotic balance, Ssk2 promotes actin cytoskeleton recovery (Yuzyuk et al. 2002). This recovery mechanism requires a polarized distribution of Ssk2, its actin-interacting activity and its kinase catalytic activity, but, interestingly, does not require Ssk1 (Yuzyuk and Amberg 2003; Bettinger et al. 2007). Although Ssk1 is the only known activator of Ssk2/Ssk22, osmostress does cause slight activation of the Hog1 MAPK in ssk1Δ sho1Δ mutants, whereas no activation is observed in ssk2Δ ssk22Δ sho1Δ mutants (Maeda et al. 1994; Reiser et al. 2000). These findings suggest that there may be an as-yet-unknown mechanism that can activate Ssk2/Ssk22 without Ssk1.
Ssk2/Ssk22-Pbs2-Hog1 kinase cascade:
Once activated, the Ssk2/Ssk22 MAPKKK initiates a kinase cascade reaction that involves the Pbs2 MAPKK and the Hog1 MAPK (Boguslawski 1992; Brewster et al. 1993). Although there are several other MAPKKs and MAPKs in yeast with similar sequences, activated Ssk2/Ssk22 exclusively phosphorylates, and thereby activates, Pbs2, and activated Pbs2 phosphorylates only Hog1. These specific interactions are due to the presence of specific docking sites in Pbs2. An Ssk2/Ssk22-specific docking site is located in the Pbs2 N-terminal regulatory region (Tatebayashi et al. 2003). Fusion of this Pbs2 docking site to the Ste7 MAPKK, which is not a substrate of Ssk2/Ssk22, allows phosphorylation of Ste7 by Ssk2/Ssk22. Pbs2 has two specific binding sites for Hog1: one is in the N-terminal regulatory region, and another is near the C terminus (Murakami et al. 2008).
The activity of wild-type Hog1 is absolutely dependent on double phosphorylation of its TGY motif by Pbs2. However, several Hog1 mutants that are partially active without any phosphorylation by Pbs2 have been isolated (Bell et al. 2001; Bell and Engelberg 2003). By using these mutants, Hog1-dependent effects can be studied without exposing cells to osmostress, which would induce both Hog1-dependent and -nondependent effects (Yaakov et al. 2003).
Stress-responsive MAPK cascades that are homologous to the Hog1 MAPK cascade are found in both lower and higher eukaryotes (Sheikh-Hamad and Gustin 2004). For example, the mammalian stress-responsive p38 MAPK is structurally highly similar to Hog1, and p38 can complement mutant strains of yeast that lack the Hog1 MAPK (Han et al. 1994). Also, the kinase domain of the mammalian stress-responsive MAPKKK termed MTK1 (also known as MEKK4) is highly similar to the kinase domains of Ssk2 and Ssk22, and expression of constitutively active MTK1-ΔN can complement the ssk2Δ ssk22Δ double mutation (Takekawa et al. 1997). MTK1 is activated by binding of its specific activator, Gadd45, in a manner similar to activation of Ssk2 and Ssk22 by Ssk1, although these activators are unrelated and not functionally exchangeable (Takekawa and Saito 1998; Mita et al. 2002; Miyake et al. 2007).
Sln1-Ypd1-Skn7 multistep phosphorelay:
Ypd1 donates its phosphoryl group not only to Ssk1 but also to Skn7 (Figure 4B). Skn7 is composed of an N-terminal DNA-binding domain and a C-terminal REC domain and is highly conserved among fungi (Brown et al. 1994). A phosphotransfer reaction from Sln1 to Skn7 via the intermediary Ypd1 was demonstrated in vitro (Li et al. 1998; Ault et al. 2002). Although Skn7 is exclusively localized in the nucleus and Ssk1 is mostly in the cytoplasm, Ypd1 is found in both the nucleus and the cytoplasm, which is consistent with its ability to transfer phosphate to both Skn7 and Ssk1 (Lu et al. 2003). The Sln1-Ypd1-Skn7 phosphorelay regulates a response that is complementary to that of the Sln1-Ypd1-Ssk1 phosphorelay: whereas Ssk1 is activated under hyperosmotic conditions, Skn7 is activated under hypo-osmotic conditions. Skn7 regulates oxidative stress-responsive genes, and skn7Δ mutants are hypersensitive to oxidative stresses such as exposure to hydrogen peroxide (Krems et al. 1996; Raitt et al. 2000a). However, the role of Skn7 in oxidative responses is not dependent on Sln1, and the phospho-accepting Asp-427 of Skn7 is not required (Morgan et al. 1997; He et al. 2009). In contrast, induction of hypo-osmostress responsive genes, such as OCH1, is dependent on Sln1 and requires the Asp-427 of Skn7 (Ketela et al. 1998; Li et al. 2002; Shankarnarayan et al. 2008). OCH1 encodes the mannosyltransferase in the cis-Golgi apparatus that initiates N-linked glycosylation of secreted/membrane proteins and thus is a key enzyme in cell-wall maintenance. Although the skn7Δ mutants are not osmosensitive, the suppression of the hypo-osmotic stress sensitivity of a pkc1Δ mutant by SKN7 overexpression suggests that Skn7 and the PKC pathway coordinately regulate cell-wall integrity that is critical for growth under hypo-osmotic conditions (Brown et al. 1994). For more details on Skn7, see a recent comprehensive review by Fassler and West (2011).
Sho1 branch of the HOG pathway
Unlike the Sln1 branch, which is a variation of the well-understood two-component paradigm, the activation mechanism of the Sho1 branch is still only vaguely defined. Although many important observations have been made, there is still a lack of a unifying mechanism that incorporates all of the separate facts. Thus, we will first present an overview of the current hypothesis of how the Sho1 branch might be activated and will then discuss the details of individual steps in the following sections.
Overview:
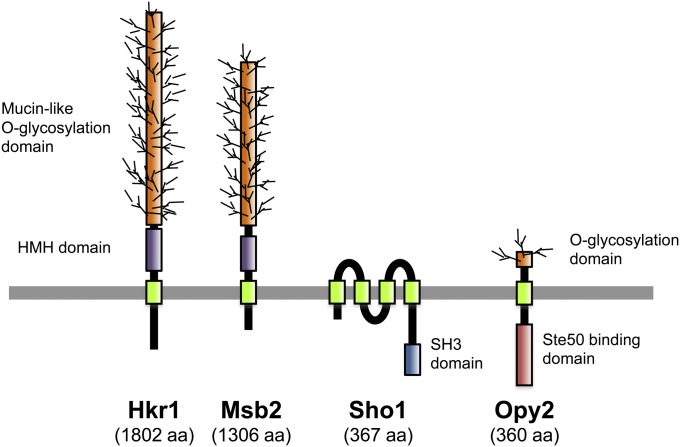
A signaling response in the Sho1 branch is initiated by the putative osmosensors Msb2 and Hkr1, which are highly glycosylated single-pass TM proteins (Tatebayashi et al. 2007). Through an as-yet-undefined mechanism that seems to involve an interaction between the Msb2/Hkr1 osmosensors and the Sho1 co-osmosensor, this response leads to activation of the PAK-like kinases Ste20 and Cla4 by inducing their association with the membrane-bound small G-protein Cdc42 (Lamson et al. 2002). Activated Ste20/Cla4 then phosphorylates and activates the Ste11 MAPKKK (Raitt et al. 2000b; van Drogen et al. 2000), which in turn phosphorylates and activates the Pbs2 MAPKK that is associated with the Sho1 membrane anchor (Maeda et al. 1995; Tatebayashi et al. 2006). Because both the Cdc42-Ste20 and the Sho1-Pbs2 complexes are localized on the membrane, Ste11 must also be localized to the membrane so that efficient activator/substrate interactions between Ste20 and Ste11, as well as between Ste11 and Pbs2, can take place. Membrane localization of Ste11 is mediated by the Ste50 adaptor protein, which forms a stable complex with Ste11 (Posas et al. 1998; Wu et al. 1999), primarily via association of Ste50 with the membrane anchor protein Opy2 (Ekiel et al. 2009; Yamamoto et al. 2010), and secondarily by Ste50–Cdc42 and Ste50–Sho1 interactions (Tatebayashi et al. 2006; Truckses et al. 2006). Activation of the Hog1 MAPK by Pbs2 seems to proceed as in the Sln1 branch.
Putative osmosensors Msb2 and Hkr1:
Both Msb2 and Hkr1 are highly glycosylated single-path transmembrane proteins (Figure 5). The extracellular domains of these proteins are highly Ser/Thr rich and contain numerous O-glycosylation sites that are glycosylated by the protein O-mannnosyl transferase Pmt4 (Yang et al. 2009). The MSB2 gene was originally identified as a multicopy suppressor of a cdc24 mutant (Bender and Pringle 1989). Since Cdc24 is a guanine exchange factor for Cdc42, it is believed that Msb2 somehow regulates the activity of Cdc24 or Cdc42. Indeed, a weak binding between Msb2 and Cdc42 has been observed (Cullen et al. 2004). However, how Msb2 controls Cdc42 activity is unclear.
Figure 5 .
Schematic representations of the four transmembrane proteins involved in the Sho1 branch of the HOG pathway. HMH, Hkr1-Msb2 homology domain. Not drawn to scale.
The possible involvement of Msb2 in the HOG pathway was initially suggested by the observation that the weak osmo-tolerance of the ssk1Δ sho1Δ mutant was abolished in the ssk1Δ sho1Δ msb2Δ triple mutant (O’Rourke and Herskowitz 2002). This observation was interpreted at that time as indicating that Msb2 is a third osmosensor in the HOG pathway (Sln1 and Sho1 being the other two). A later study, however, revealed that Msb2 and another transmembrane glycoprotein, Hkr1, are the more likely osmosensors in the Sho1 branch, but that Sho1 itself has a downstream function as a co-osmosensor (Tatebayashi et al. 2007). This conclusion is partly based on genetic epistasis tests that indicated that MSB2/HKR1 functions upstream of SHO1: a constitutively active SHO1 mutant can activate Hog1 MAPK even in the msb2Δ hkr1Δ double-mutant cells, but a constitutively active MSB2 or HKR1 mutant cannot activate Hog1 in a sho1Δ mutant.
The Ser/Thr-rich glycosylation domains of Msb2 and Hkr1 have a negative regulatory function, as their deletion converts Msb2 and Hkr1 into constitutively active forms (Cullen et al. 2004; Tatebayashi et al. 2007). Furthermore, inhibition of O-glycosylation by pmt4Δ mutation, together with inhibition of N-glycosylation by tunicamycin, activates the Hog1 MAPK cascade in an Msb2-dependent manner (Yang et al. 2009). Based on these observations, two possible mechanisms of activating these osmosensors have been proposed. One is by proteolytic cleavage in the extracellular domain by the aspartyl protease Yps1, which eliminates the Ser/Thr-rich glycosylation domain (Vadaie et al. 2008). Another is by an osmostress-induced conformational change in the oligosaccharide structure (Tatebayashi et al. 2007). However, the actual mechanism remains unclear.
Co-osmosensor Sho1:
The SHO1 gene was initially identified by isolation of mutants that are synthetically high osmolarity sensitive in the presence of mutations that inactivate the Sln1 branch of the HOG pathway (Maeda et al. 1995). Sho1 is a relatively small protein (367 aa) that is composed of an N-terminal bundle of four transmembrane segments (TM1–TM4) and a C-terminal, cytoplasmic SH3 domain (Figure 5). The Sho1 SH3 domain binds to a Pro-rich motif (KPLPPLPV) in the N-terminal regulatory region of Pbs2 and serves to localize Pbs2 to the membrane (Maeda et al. 1995). Of the 27 SH3 domains found in the yeast proteome, only the Sho1-SH3 binds Pbs2, indicating a very high level of selectivity (Zarrinpar et al. 2003). The Sho1–Pbs2 interaction is required for activation of Pbs2 by the Ste11 MAPKKK. The Sho1 SH3 domain can also bind to Pro-rich motifs in Fus1 (KPLPLTPN) (Nelson et al. 2004) and in Ste20 (QPLPPIPP) (K. Tanaka, K. Tatebayashi, H.-Y. Yang, and H. Saito, unpublished results). Thus, during a mating response, induced Fus1 might downregulate the Sho1 branch by competitively inhibiting the Sho1–Pbs2 interaction. The role of the Sho1–Ste20 interaction seems to be redundant with that of other signaling elements in the Sho1 branch because this Pro-rich motif in Ste20 is required for activation of the Sho1 branch only in some mutants, but not in wild-type cells.
A few lines of evidence suggest that Sho1 might serve additional roles in signaling other than membrane targeting of Pbs2 and Ste20. First, Pbs2 appears to dissociate from Sho1 upon activation of the Pbs2 MAPKK, as suggested by decreased membrane localization of Pbs2 following osmostress stimulation, and this dissociation is hindered in a ste20Δ or a ste11Δ mutant or in a pbs2Δ mutant in which a catalytically inactive Pbs2-K389M is expressed, but interestingly not in a hog1Δ mutant (Reiser et al. 2000). These observations suggest that the Sho1–Pbs2 interaction might be dynamically regulated by a feedback phosphorylation by activated Pbs2. Second, an experimental replacement of the Sho1 SH3 domain with another SH3 domain derived (and modified) from the Fyn kinase resulted in a hybrid Sho1 that bound to Pbs2 just as well as the wild-type Sho1. Nonetheless, such a hybrid Sho1 is functionally defective, implying that the Sho1 SH3 domain has other functions in addition to Pbs2 binding (Marles et al. 2004). Third, and possibly related to the previous point, several proteins, notably Ste11 and Ste50, have been shown to interact with Sho1, but this binding is independent of the Pro-rich-motif-binding ability of the Sho1 SH3 domain (Zarrinpar et al. 2004; Tatebayashi et al. 2006). These interactions might enable Ste11 to efficiently interact with Pbs2 that is associated with Sho1. Finally, there are a number of Sho1 mutants that are constitutively activated in the sense that their expression will activate the Hog1 MAPK in the absence of any osmostress (Tatebayashi et al. 2006, 2007; Vadaie et al. 2008). These mutations are found both in the TM region and in the cytoplasmic region, suggesting that Sho1 might engage in dynamic interaction with other molecules through both its TM and cytoplasmic regions. Thus, the potentially dynamic functions of Sho1 are still far from being understood.
Adaptor protein Ste50:
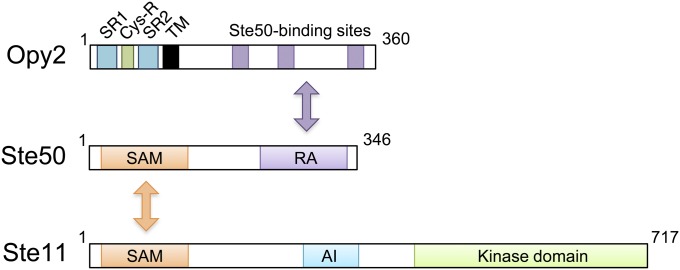
STE50 was originally identified as a gene that is required for an efficient mating response, as its deletion mutants are moderately sterile (Ramezani Rad et al. 1992; Xu et al. 1996). Ste50 is essential for the Sho1 branch of the HOG pathway (Posas et al. 1998; Wu et al. 1999) and is also necessary for the filamentous and invasive growth pathway that activates the Kss1 MAPK (Ramezani Rad et al. 1998; Jansen et al. 2001). Thus, all three signal pathways that involve Ste11 are dependent on Ste50. Structurally, Ste50 is composed of an N-terminal sterile-α motif (SAM) domain and a C-terminal Ras association (RA) domain (Ramezani-Rad 2003) (Figure 6). A SAM domain is a protein interaction module of ∼70 amino acids that can homo-dimerize and hetero-oligomerize with other SAM domains (Qiao and Bowie 2005). In vivo binding studies have shown that the Ste50 SAM domain binds to the SAM domain in Ste11 (Posas et al. 1998; Wu et al. 1999; Jansen et al. 2001), while in vitro studies demonstrated that the Ste50 SAM domain can homo-dimerize as well as hetero-dimerize with Ste11 SAM (Bhattacharjya et al. 2004; Grimshaw et al. 2004; Kwan et al. 2004, 2006). The SAM-mediated Ste50–Ste11 interaction is essential for all the known activities of Ste50 (Ramezani-Rad 2003).
Figure 6 .
Schematic diagram of the Ste11/Ste50/Opy2 complex. Ste11 and Ste50 bind together through their SAM domains, whereas the RA domain of Ste50 binds to any of three binding sites in Opy2. AI, autoinhibitory domain; Cys-R, cysteine-rich domain; SR, Serine rich domain; TM, transmembrane domain.
In spite of its name, the Ste50 RA domain does not seem to interact with Ras proteins. Genetic evidence suggests that the RA domain might interact with the Cdc42 GTPase, which is supported by a coprecipitation assay that showed that the Ste50 RA domain interacted equivalently with either GTP- or GDP-bound Cdc42 (Tatebayashi et al. 2006; Truckses et al. 2006). A Ste50 mutant that lacks the RA domain (Ste50-ΔRA) is functionally defective and cannot activate the Hog1 MAPK in response to osmostress. However, forced localization of Ste50-ΔRA to the plasma membrane, by attachment of a membrane-targeting signal, results in efficient activation of the Hog1 MAPK, indicating that an essential function of the RA domain is to aid Ste50 membrane localization (Tatebayashi et al. 2006; Truckses et al. 2006; Wu et al. 2006). In wild-type cells, Ste50 membrane localization could be attained, in principle, by an interaction of the Ste50 RA domain with the membrane-associated Cdc42 GTPase. However, the major factor that recruits Ste50 to the membrane appears to be the membrane anchor protein Opy2 (Wu et al. 2006; Yamamoto et al. 2010). Importantly, membrane-targeting of Ste50-ΔRA, using the Ras C-terminal prenylation signal, can rescue the osmostress-induced Hog1 activation in the absence of Opy2, implying that the Ste50–Opy2 interaction and resulting Ste50 membrane localization is the main function of the Ste50 RA domain (Tatebayashi et al. 2007). Ste50 has also been shown to interact with the membrane protein Sho1 (Tatebayashi et al. 2006), but the roles of Ste50–Sho1 interaction in signaling remain to be determined. In summary, the main function of Ste50 seems to be to serve as an adaptor between the Ste11 MAPKKK and the membrane anchor Opy2, so that Ste11 is efficiently recruited to the membrane.
Membrane anchor Opy2:
The OPY2 gene was initially identified as a multicopy suppressor that downregulates the mating MAPK signal pathway (Edwards et al. 1997). However, disruption of OPY2 does not have any significant impact on the mating pathway. It was later found that the opy2Δ mutation, together with a defect in the Sln1 branch, causes synthetic osmosensitivity, indicating that Opy2 has an essential function in the Sho1 branch of the HOG pathway (Wu et al. 2006).
Opy2 is a single-path transmembrane protein of 360 aa. Its short extracellular domain is composed of, from the N terminus, a highly Ser-rich (SR1) domain, a Cys-rich (Cys-R) domain, and another Ser-rich (SR2) domain followed by the TM segment (Figure 6). The SR1 domain, but not SR2, is highly O-glycosylated by the protein O-mannnosyl transferase Pmt4, but deletion of SR1 does not have any observable effect on Opy2 functions (Hutzler et al. 2007; Yang et al. 2009). The Cys-R domain is characterized by an arrangement of eight cysteine residues, and genes that encode a similar Cys-rich motif are found in a wide range of fungal species. The cytoplasmic region of Opy2 is intrinsically disordered as revealed by NMR spectroscopy (Ekiel et al. 2009) and comprises four short well-conserved regions (CR-A to CR-D) interspersed among nonconserved sequences (Yamamoto et al. 2010).
The essential function of Opy2 in the Sho1 branch is to recruit the Ste50/Ste11 complex to the plasma membrane. Earlier studies suggested that there is more than one Ste50-binding site in Opy2 (Wu et al. 2006; Ekiel et al. 2009). A more recent study extended this hypothesis and showed that there are actually three independent Ste50-binding sites in Opy2, which correspond to the conserved regions CR-A, CR-B, and CR-D. CR-A and CR-D seem to constitutively bind Ste50, whereas CR-B (DIRSHITLGSSIL) binds Ste50 only when the Ser and Thr residues are phosphorylated by the casein kinase I isoforms, Yck1 and Yck2 (Yamamoto et al. 2010). Yck1/Yck2 are activated when glucose availability is high (Zaman et al. 2008). In fact, Opy2 CR-B is phosphorylated only when there is abundant glucose in the media. Opy2 is required not only for the Sho1 branch, but also for the FIG pathway, which is activated under limited nutrition and activates the Kss1 MAPK. Interestingly, CR-B seems to function only in the Hog1 pathway, but not in the FIG pathway. Thus, it is possible that under glucose-rich environments the phosphorylation of CR-B shifts Opy2 activity away from Kss1 and toward Hog1.
In summary, the main function of Opy2 is to serve as a membrane anchor for the Ste11 MAPKKK through its binding to the adaptor protein Ste50. Opy2 also integrates signals from the osmosensors and the glucose sensors.
Activation of Ste20/Cla4:
Ste20 is a member of the p21-activated kinase (PAK) family of protein kinases that are activated by the small GTPase Cdc42 (Bokoch 2003). In the absence of stimuli, PAK family kinases are inhibited by their N-terminal auto-inhibitory domain that binds to their C-terminal kinase domain (Lei et al. 2000). This auto-inhibition is relieved when GTP-bound (activated) Cdc42 binds to the p21-binding domain termed “CRIB” that is close to the auto-inhibitory domain (Peter et al. 1996; Leberer et al. 1997; Lamson et al. 2002; Ash et al. 2003). Ste20 was initially identified as a kinase that is required to activate the Ste11 MAPKKK in the mating signal pathway (Leberer et al. 1992). Later, Ste20 was shown to participate in two other signal pathways, the FIG and the Sho1 branch of the HOG pathway (Mösch et al. 1996; O’Rourke and Herskowitz 1998; Raitt et al. 2000b). Cla4 is another PAK family kinase and is involved mainly in cell-cycle regulation, such as septin formation and polarized growth (Tjandra et al. 1998). Although both ste20Δ and cla4Δ mutants are viable, the ste20Δ cla4Δ double mutation is lethal (Cvrcková et al. 1995). Thus, it is believed that Ste20 and Cla4 share at least one essential function, although the nature of that essential function is not known.
The growth of ste20Δ mutants of a parental strain that is defective in the Sln1 branch, such as ssk2Δ ssk22Δ, is sensitive to high osmolarity, but these mutants can tolerate moderate osmostress (Raitt et al. 2000b). In contrast, ste20Δ cla4ts double mutants of the same strain are highly osmosensitive and are completely unable to activate Hog1, indicating that Cla4 partially compensates for the function of Ste20 (Tatebayashi et al. 2006). The finding that ste20(ΔCRIB) mutants are more osmosensitive than the STE20 wild-type parental cells seems to indicate that Cdc42 binding to Ste20 is required for activation and/or membrane localization of Ste20 (Raitt et al. 2000b; Winters et al. 2005). However, overexpression of constitutively active cdc42(G12V) only very moderately activates Hog1, suggesting that an additional factor might be necessary for full activation of Ste20 (Raitt et al. 2000b). Although it is frequently assumed that GTP association of Cdc42 is increased and that Ste20 kinase is activated in response to osmostress, there is no direct evidence for these assumptions. An alternative mechanism, in which osmostress induces the association of active Ste20 (which has been activated by an osmostress-independent manner) and Ste11, might better fit the available data. Indeed, the mating MAPK pathway is activated by an analogous mechanism, i.e., by pheromone-induced association of Ste20 and Ste11 (Pryciak and Huntress 1998; Lamson et al. 2002).
Activation of Ste11 by Ste20/Cla4:
Activation of the Ste11 MAPKKK by osmostress requires at least two events. The first event is the binding of Ste50 to the Ste11 N-terminal SAM domain. This interaction helps to dissociate the N-terminal inhibitory domain from the C-terminal kinase catalytic domain, thus relieving inhibition of the kinase (Wu et al. 1999). However, as the Ste11–Ste50 interaction is constitutive, this effect is not likely to play an active role in regulating Ste11 activity during osmostress. The second event that is required is phosphorylation of Ste11 by Ste20/Cla4. It has been demonstrated that, in response to α-mating factor, activated Ste20 phosphorylates Ser-302, Ser-306, and Thr-307 in the N-terminal regulatory region of Ste11 (van Drogen et al. 2000). Based on the effects of phospho-mimetic mutations, it is believed that these Ste11 sites are also phosphorylated by Ste20/Cla4 upon osmostress stimulation (Lamson et al. 2006).
Ste50 binding and phosphorylation by Ste20/Cla4 are important, but not sufficient for Ste11 to transmit signals to downstream elements. Phospho-mimetic substitutions at the phosphorylation sites, or mutations in the auto-inhibitory domain, or even a deletion of the entire N-terminal regulatory region, all constitutively activate Ste11. Overexpression of one of these constitutively active Ste11 mutants activates both the Ste11-Pbs2-Hog1 and the Ste11-Ste7-Fus3/Kss1 MAPK cascades, without any stimulation (Posas and Saito 1997; Lamson et al. 2006; Tatebayashi et al. 2006). However, expression of the same constitutively active Ste11 mutants using the native STE11 promoter does not significantly activate the Hog1 MAPK or the Fus3/Kss1 MAPK (Lamson et al. 2006; Tatebayashi et al. 2006). Constitutively active Ste11 mutants do activate the Hog1 MAPK cascade and the mating MAPK cascade in a Ste20/Cla4-independent manner upon respective stimulation (Lamson et al. 2006; Tatebayashi et al. 2006). Thus, it is clear that, in addition to activation of Ste11 by Ste20/Cla4, another stimulus-dependent signal amplification step is required to transmit sufficient signal to the downstream component (Pbs2 in the case of the HOG pathway and Ste7 in the cases of the mating and FIG pathways). The nature of this amplification step is unclear, but one possibility is a stimulus-induced membrane localization of activated Ste11 (Lamson et al. 2006).
Activation of Pbs2 by Ste11:
Ste11 can be activated by any of the three MAPK cascades: the osmoregulatory HOG pathway, the mating pathway, and the FIG pathway. When activated by osmostress, however, Ste11 activates only the Pbs2 MAPKK, while in the other pathways Ste11 activates the Ste7 MAPKK. Thus, there must be a mechanism that allows only Pbs2 to be activated by Ste11 during osmotic stimulation. As discussed earlier, Pbs2 is recruited to the plasma membrane by the membrane-associated scaffold protein Sho1 (Maeda et al. 1995; Reiser et al. 2000), and the Ste11/Ste50 complex is recruited to the membrane by the membrane anchor protein Opy2 (Wu et al. 2006; Ekiel et al. 2009; Yamamoto et al. 2010). However, efficient activation of Pbs2 by Ste11 seems to require, in addition to their membrane localization, direct and indirect docking interactions between Ste11 and Pbs2. It is known that Ste11 and Pbs2, Ste11 and Sho1, Ste50 and Sho1, and possibly Opy2 and Sho1 bind to each other (Posas and Saito 1997; Zarrinpar et al. 2004; Tatebayashi et al. 2006). Thus, multiple interactions between the Opy2/Ste50/Ste11 complex and the Sho1/Pbs2 complex bring Ste11 in close contact with Pbs2 for efficient activation. The relative contributions of these interactions to Pbs2 activation, as well as their regulation by osmostress, remain to be determined.
Activation of the HOG pathway by non-osmotic stresses
A number of non-osmotic stresses are known to activate the HOG pathway, including cold stress (Hayashi and Maeda 2006; Panadero et al. 2006), heat stress (Winkler et al. 2002), hypoxia (Hickman et al. 2011), arsenite (Sotelo and Rodríguez-Gabriel 2006; Thorsen et al. 2006), acetic acid (Mollapour and Piper 2006, 2007), low pH (Kapteyn et al. 2001), inhibition of glycosylphosphatidylinositol (GPI) anchor synthesis (Toh-E and Oguchi 2001), and inhibition of sphingolipid synthesis (Tanigawa et al. 2012). In most cases, Hog1 is only moderately activated, and the kinetics of Hog1 phosphorylation is different from those observed upon osmostress. Although it is unclear how Hog1 is activated by these stresses, such stresses often activate either the Sln1 branch or the Sho1 branch, but not both. Adaptation to these diverse stresses, in addition to osmostress, might explain why yeast has apparently redundant osmostress-signaling branches. In this context, it is worth noting that the Aspergillus nidulans HogA MAPK (a homolog of Hog1) is activated only by the two-component signaling pathway homologous to the Sln1 branch, even though the mold has a Sho1 homolog (Furukawa et al. 2005).
Nuclear transport of activated Hog1
Hog1 rapidly accumulates in the nucleus following osmotic stress (Figure 7A). Hog1 is then exported back to the cytoplasm after return to an iso-osmotic environment or after adaptation to high osmolarity (Ferrigno et al. 1998; Reiser et al. 1999). The kinetics of the transient Hog1 nuclear localization closely correlate with those found for the dual phosphorylation of Hog1 at Thr-174 and Tyr-176 (Figure 7B). Indeed, Hog1 mutations at these amino acid positions prevent Hog1 translocation into the nucleus (Ferrigno et al. 1998; Reiser et al. 1999). Hog1 phosphorylation itself, however, is not sufficient for its nuclear localization because the constitutively phosphorylated Hog1 molecules in the ptp2Δ ptc1Δ double-mutant cells do not accumulate in the nucleus (Mattison and Ota 2000). Catalytically inactive Hog1 mutants, such as D144A, cannot translocate into the nucleus after hyper-osmotic stimulation (Westfall and Thorner 2006). In contrast, other catalytic site mutants that retain partial activity, such as K52R or K52M, not only translocate into the nucleus, but also even fail to be exported out of the nucleus (Ferrigno et al. 1998; Mattison and Ota 2000). Thus, Hog1 catalytic activity seems to be required for its nuclear import and/or export, but its precise role remains unclear. Strains that lack the general stress activators Msn2 and Msn4, the related transcription factors Msn1 and Hot1, or the nuclear protein tyrosine phosphatase Ptp2 accumulate less Hog1 in the nucleus than wild-type cells, suggesting that these molecules bind and retain Hog1 in the nucleus (Reiser et al. 1999; Rep et al. 1999b; Mattison and Ota 2000).
Figure 7 .
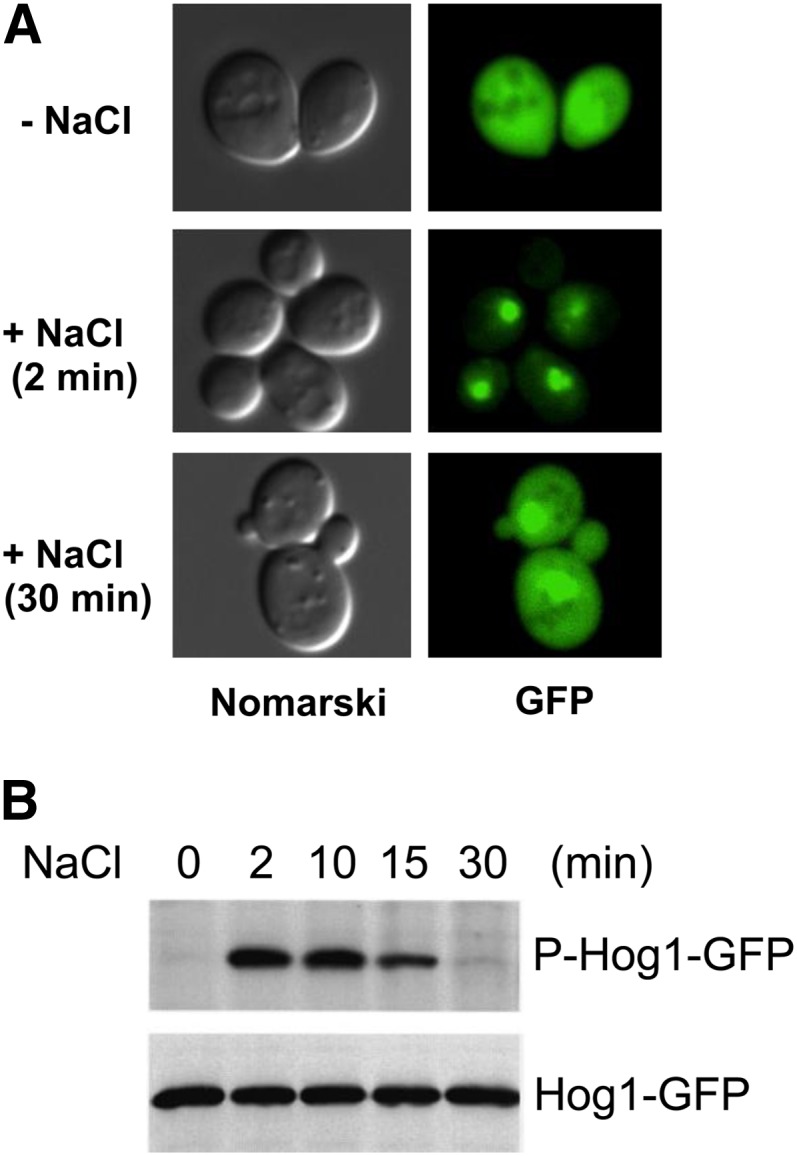
Transient phosphorylation and nuclear localization of the Hog1 MAPK after osmostress. GFP-tagged Hog1 (Hog1-GFP) was expressed in a hog1Δ host strain, and cells were exposed to 0.4 M NaCl for the time indicated. (A) Hog1-GFP was detected by fluorescence microscopy (GFP), while the cell shape was pictured by differential interference contrast microscopy (Nomarski). (B) Total Hog1-GFP and phosphorylated Hog1-GFP were detected by immunoblotting using, respectively, anti-GFP and anti-phosphotyrosine antibody. Modified from Ferrigno et al., 1998.
Nuclear import of Hog1 is partially dependent on the activity of Gsp2 (homolog of mammalian Ran GTPase) and Nmd5 (homolog of importin β), but not on that of Srp1 and Rsl1, which encode the nuclear localization signal (NLS)-binding importin α/β heterodimer (Ferrigno et al. 1998). This result is consistent with the fact that Hog1 does not contain a classical NLS. Nuclear export of Hog1 requires the activity of the nuclear export signal (NES) receptor Xpo1/Crm1 (Ferrigno et al. 1998).
Nuclear localization is necessary for Hog1 to phosphorylate its nuclear substrates, including transcription factors and cell-cycle regulators. Indeed, cells that express plasma membrane-tethered Hog1 (Hog1-CCAAX), which cannot translocate to the nucleus, seem to have deficient expression of the Hog1-dependent genes (Westfall et al. 2008). Strikingly, however, membrane-tethered Hog1 permits robust growth under conditions of hyper-osmotic stress, suggesting that Hog1-mediated cytoplasmic modulation of metabolic activities, perhaps those that are necessary for glycerol synthesis and accumulation, are more important for long-term cell survival than alteration of the gene expression pattern (Bouwman et al. 2011).
Unlike Hog1, the Hog1-activating kinase Pbs2 is found mostly in the cytoplasm of both unstressed and osmostress-stimulated cells (Ferrigno et al. 1998). Nevertheless, Pbs2 has an NES at its N terminus (residues 4–18) and an NLS at its C terminus (residues 636–639). Pbs2 ΔNES mutants accumulate in the nucleus, whereas Pbs2 ΔNES ΔNLS double mutants are found in the cytoplasm (Tatebayashi et al. 2003). Thus, it is likely that Pbs2 shuttles between the two compartments, but the function of such shuttling is unknown.
Dynamics of HOG pathway signaling
The Hog1 MAPK is only transiently activated following osmostress stimulation. Phosphorylation of the Hog1 activation sites (TGY) increases rapidly, reaches a maximal level at ∼5 min, and then gradually decreases to near basal levels within 30 min (Maeda et al. 1995; Hao et al. 2007) (Figure 7B). This negative regulation is dependent on the kinase activity of Hog1 itself because phosphorylation of catalytically inactive Hog1 persists much longer than that of wild-type Hog1 (Wurgler-Murphy et al. 1997). Several negative-feedback mechanisms are known in the HOG pathway. Furthermore, the Hog1 MAPK pathway is part of a complex signaling network that involves at least two other MAPK pathways. The dynamic characteristics of this signal network are intensely investigated both by conventional genetic/biochemical approaches and by more recent systems biological and computational approaches.
Negative feedback by glycerol accumulation:
The most important negative feedback mechanism of Hog1 pathway signaling is removal of the osmostress by induced accumulation of the compatible solute glycerol (Brewster et al. 1993; Albertyn et al. 1994; Klipp et al. 2005; Muzzey et al. 2009). Although transcriptional induction of GPD1 and other genes necessary for glycerol accumulation is important for long-term downregulation of the Hog1 pathway, such induction takes too long (at least 15 min) to account for the rapid decline of Hog1 activity (Hirayama et al. 1995). It has been proposed that Hog1 might more rapidly regulate glycerol accumulation by directly modulating the activities of the glycerol channel Fps1 and metabolic enzymes involved in glycerol biosynthesis (Dihazi et al. 2004; Klipp et al. 2005; Mollapour and Piper 2007; Westfall et al. 2008; Beese et al. 2009; Bouwman et al. 2011).
Negative feedback by protein phosphatases:
Although signaling from the upstream osmosensors stops when osmotic imbalance is eliminated by glycerol accumulation, it is still necessary to inactivate the kinases by dephosphorylation to bring the system to the prestimulation state. The two activating phosphorylation sites in Hog1, namely Thr-174 and Tyr-176, are dephosphorylated by different enzymes (for reviews, see Saito and Tatebayashi 2004; Martín et al. 2005).
Members of the type 2C Ser/Thr phosphatase family, Ptc1, Ptc2, and Ptc3, dephosphorylate Thr-174. Of these phosphatases, Ptc1 is the most important for de-activation of Hog1, as the ptc1Δ mutant retains high Hog1 activity even after 1 hr (Warmka et al. 2001). The specificity of Ptc1 toward Hog1 is indirectly conferred by the adaptor protein Nbp2 (Mapes and Ota 2004). Nbp2 binds to both Ptc1 and Pbs2, and as Pbs2 also has a high affinity for Hog1, Ptc1 is indirectly recruited to Hog1 by the Nbp2–Pbs2 complex. In contrast, Ptc2 and Ptc3 seem to have more of a subsidiary role of limiting the maximal activity of Hog1 during activation (Young et al. 2002).
Members of the protein tyrosine phosphatase family, Ptp2 and Ptp3, dephosphorylate Tyr-176 (Jacoby et al. 1997; Wurgler-Murphy et al. 1997). Although these tyrosine phosphatases are partially redundant, Ptp2 is primarily responsible for Hog1 dephosphorylation, whereas Ptp3 is more important for Fus3 dephosphorylation (Zhan and Guan 1999). Ptp2 is found in the nucleus, whereas Ptp3 is localized in the cytoplasm (Mattison and Ota 2000). This localization of Ptp2 seems to ensure that tyrosine dephosphorylation of Hog1 occurs only after Hog1 has entered into the nucleus. Because Hog1 is inactivated when either Thr-174 or Tyr-176 is dephosphorylated, the ptc1Δ ptp2Δ double-mutant strain is lethal because of Hog1 hyperactivation (Maeda et al. 1993). Phosphatases that inactivate other kinases in the Hog1 pathway have not been identified confidently.
Negative feedback by phosphorylation of upstream elements:
Activated Hog1 also negatively feedback regulates the Hog1 pathway by phosphorylating upstream signaling elements. Osmostress-activated Hog1 phosphorylates Sho1 at Ser-166, which is located within the cytoplasmic linker region between the four TM domains and the C-terminal SH3 domain (Hao et al. 2007). Hog1 activation is slightly diminished in cells expressing the phosphomimetic Sho1-S166E. It has been shown that some mutations at Ser-166 disrupt Sho1 oligomerization. However, neither the role of Ser-166 phosphorylation in Sho1 oligomerization, nor the role of Sho1 oligomerization in Hog1 activation, is clear.
Activated Hog1 phosphorylates several amino acids in Ste50 (Ser-155, Ser-196, Ser-202, Thr-244, Ser-248, and Thr-341) (Hao et al. 2008). Phosphorylation of Ste50 reduces its affinity for the membrane anchor Opy2 (Yamamoto et al. 2010). Because the Opy2–Ste50 interaction is essential for Hog1 activation via the SHO1 branch, phosphorylation of Ste50 by Hog1 serves as a negative feedback mechanism. Indeed, the duration of Hog1 activation by osmotic stress is longer in cells that express a phosphorylation-deficient Ste50 mutant than in the control cells. Pheromone-activated Fus3 and Kss1 also phosphorylate the same Ste50 residues, suggesting that Ste50 phosphorylation may also serve as a cross-regulatory mechanism between the mating and HOG pathways (Yamamoto et al. 2010).
Inhibition of crosstalk among MAPK signaling pathways:
In general, each MAPK module is activated by specific types of stimuli and induces specific adaptive responses. To achieve this specificity would be easy if each MAPK module was composed of only unique and dedicated components. In yeast, however, three MAPK modules (the Sho1 branch of HOG pathway, the mating pathway, and the FIG pathway) share many components, including the Ste11 MAPKKK, and still maintain their individuality. Leakage of signal, or crosstalk, from one MAPK pathway to another is prevented by a number of mechanisms, in addition to the negative regulation that involves protein phosphatases (Saito 2010).
One mechanism is insulation of each MAPK pathway from the others by docking interactions and scaffold proteins (Reményi et al. 2005; Bardwell 2006; Dard and Peter 2006). Activation of the mating MAPK module (Ste11→Ste7→Fus3) is dependent on the presence of the Ste5 scaffold (Elion 2001; Flatauer et al. 2005; Winters et al. 2005; Garrenton et al. 2006; Good et al. 2009). In contrast, activation of the Sho1 branch of the Hog1 MAPK module (Ste11→Pbs2→Hog1) is dependent on the presence of the Sho1 scaffold (Maeda et al. 1995; Zarrinpar et al. 2004). Indeed, when a wild-type cell is costimulated with osmostress and a mating factor, dual activation of the HOG and the mating MAPK pathways occurred, indicating that these two MAPK modules are practically insulated and activated independently of each other (Patterson et al. 2010). The importance of docking and scaffold interactions in determining pathway specificity has also been demonstrated by artificially forcing interaction between non-native pairs of signaling elements, thus diverting the signaling flow into preselected directions (Harris et al. 2001; Park et al. 2003; Tatebayashi et al. 2003; Mody et al. 2009).
Another mechanism is cross-inhibition by one MAPK pathway of other MAPK pathways. Although the Hog1 MAPK module (Ste11→Pbs2→Hog1) shares many upstream components with the FIG Kss1 MAPK module (Ste11→Ste7→Kss1), osmostress activates the Kss1 MAPK of the FIG pathway only very weakly and transiently (Shock et al. 2009; Wang et al. 2009), and glycosylation defects that activate Kss1 do not activate Hog1 (Cullen et al. 2000; Yang et al. 2009). In the absence of Pbs2 or Hog1, however, osmostress activates Kss1 robustly and Fus3 to a lesser degree, induces Kss1/Fus3-dependent genes, and induces FIG/mating-like polarized cell growth (O’Rourke and Herskowitz 1998, 2004; Pitoniak et al. 2009). Using an ATP analog-sensitive Hog1 mutant, it was shown that inhibition of this crosstalk requires Hog1 kinase activity (Westfall and Thorner 2006). Although it is possible that a part of this crosstalk inhibition is achieved by modulation of FIG/mating-specific gene expression in the nucleus (Shock et al. 2009), even a membrane-tethered version of Hog1, which, in principle, cannot enter the nucleus, can prevent this crosstalk, implying that a cytoplasmic substrate might be involved in this process (Westfall et al. 2008). However, cells expressing mutants of the known or suspected Hog1 substrate proteins (Sho1, Ste50, Opy2, Ste7, Tec1, Dig1/Dig2, and Rck1/Rck2) that lack Hog1-dependent phosphorylation sites do not display constitutive crosstalk (Hao et al. 2007, 2008; Shock et al. 2009; Yamamoto et al. 2010). Thus, the mechanism of cross-inhibition between the HOG and FIG/mating pathways remains obscure.
Single-cell dynamics:
Conventional methods used to detect MAPK activity such as immunostaining of fixed cells or immunoblotting of cell extracts using phospho-MAPK-specific antibodies can show only static snapshots and/or population averages of MAPK activation. To study the systems dynamics of a signaling pathway, it is necessary to monitor the behavior of single cells under controlled environmental conditions. The Hog1 MAPK pathway is particularly suited for this type of analysis. By using a microfluidic device to change the osmolarity of media (input), and by monitoring the nuclear translocation of fluorescent protein-tagged Hog1 (output), two groups have reported the frequency responses of HOG pathway activation (Hersen et al. 2008; Mettetal et al. 2008). At low frequency (<1/200 sec−1), the HOG pathway faithfully follows the input changes, whereas at higher frequency, it responds only to the average input osmolarity. Other aspects of HOG-signaling properties have also been studied using various single-cell monitoring methods (McClean et al. 2007; Muzzey et al. 2009; Patterson et al. 2010; Pelet et al. 2011).
In silico simulation:
The HOG-signaling pathway is also an intense subject of in silico simulation, or mathematical modeling, that aims to elucidate system architecture, dynamics, and regulation based on data sets in the literature. Modeling is rapidly evolving from a simple tool that describes and summarizes the known facts into a more advanced predictive facility that can test the validity of various hypotheses (Klipp et al. 2005; Gat-Viks and Shamir 2007; Zou et al. 2007; Krantz et al. 2009; Rensing and Ruoff 2009; Zi et al. 2010; Parmar et al. 2011; Schaber et al. 2011). The popularity of the HOG pathway for such studies is undoubtedly because of its relative simplicity together with the availability of detailed mechanistic knowledge regarding this pathway and abundant quantitative and qualitative data. Thus, the HOG pathway will continue to be an excellent testing ground for algorithms that attempt to simulate and analyze more complex signal transduction networks in higher eukaryotes.
Downstream Adaptive Responses
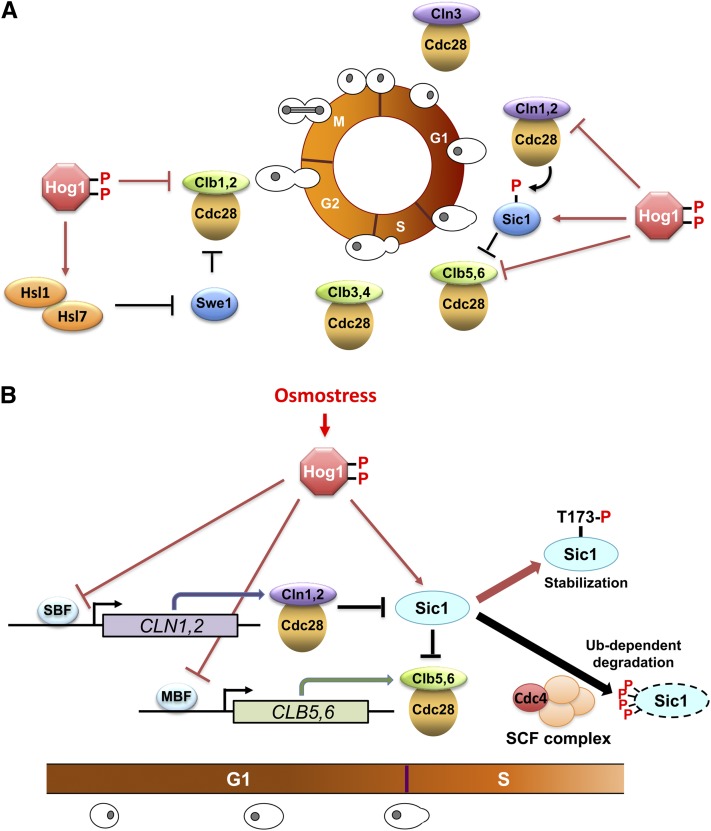
Reestablishment of osmotic balance
Compatible osmolytes:
Activation of Hog1 in response to osmostress elicits a program for cell adaptation that includes short- and long-term responses. Long-term adaptation involves transcriptional and translational regulation of the genome, whereas short-term adaptation is accomplished by changes in glycerol accumulation (Albertyn et al. 1994) and the reestablishment of ionic balance (Proft and Struhl 2004). Exposure to increased osmolarity is known to result in loss of water, shrinkage in cell size, and a temporary arrest of growth until adaptation occurs. The major strategy for survival under high osmolarity is to produce and accumulate compatible osmolytes such as glycerol to maintain the water balance and reestablish the volume and the turgor of the cells (Blomberg and Adler 1989; Hohmann et al. 2007; Westfall et al. 2008; de Nadal et al. 2011). The accumulation of compatible osmolytes is a ubiquitous mechanism in cellular osmoregulation. Although there are a number of compatible osmolytes such as trehalose, amino acids, and ions that contribute differently to adaptation to osmostress, glycerol seems to be the most important compatible osmolyte for the growth of S. cerevisiae in the presence of high osmolarity (Hohmann et al. 2007).
Intracellular accumulation of glycerol is an essential response for survival under high-osmolarity conditions, and the Hog1 MAPK is responsible mainly for the accumulation of glycerol in the presence of high osmolarity (Albertyn et al. 1994). There are several mechanisms to control glycerol accumulation: regulation of gene expression, metabolic adjustment, and control of glycerol export and import (Hohmann 2002b).
Glycerol accumulation:
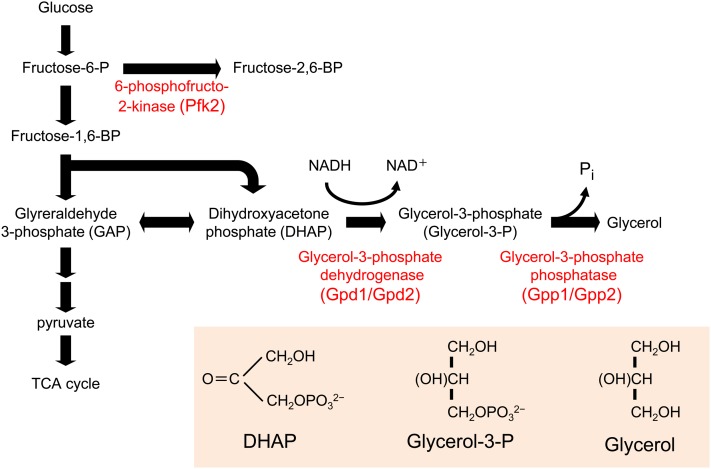
The expression of key metabolic enzymes that are involved in glycerol, trehalose, and glycogen metabolism is upregulated in response to Hog1 activation. The enzymes directly responsible for the synthesis of glycerol, i.e., glycerol-3-phosphate dehydrogenase (Gpd1) and glycerol-3-phosphatases (Gpp1 and Gpp2), are upregulated upon osmostress (see below), and the lack of these genes severely impairs growth at high osmolarity (Figure 8) (Hohmann 2002a). Expression of sugar transporters and genes involved in sugar metabolism are also upregulated in response to osmostress (Rep et al. 1999a, 2000; Gasch et al. 2000; Tomás-Cobos et al. 2004; Capaldi et al. 2008). However, some studies indicated that regulation of gene expression by Hog1 is not absolutely required for cell survival under certain high-osmolarity conditions, especially at the initial phases of the stress and at medium osmolarity (Mettetal et al. 2008; Westfall et al. 2008). In contrast, other studies indicated that Hog1-dependent regulation of the expression of specific genes involved in glycerol metabolism is important for cell survival at high osmolarity over an extended period of time (Hohmann 2002b; de Nadal and Posas 2010; Martínez-Montañés et al. 2010).
Figure 8 .
Glycerol biosynthetic pathway. Glycerol is synthesized from an intermediate in the glycolysis, dihydroxyacetone phosphate (DHAP), by two-step enzymatic reactions. The first enzyme is glycrol-3-phosphate dehydrogenase (Gpd1/Gpd2), which reduces DHAP using NADH as reducing agent. The second enzyme is glycerol-3-phosphate phosphatase (Gpp1/Gpp2), which removes phosphate from glycerol-3-P to generate glycerol.
Glycerol is rapidly accumulated in response to osmostress, starting within the first minute, and there is significant accumulation of glycerol after 30 min of exposure to high osmolarity (Klipp et al. 2005). This rapid increase in glycerol production cannot be attributed to an increase in the transcription of particular genes, and therefore additional mechanisms other than transcriptional regulation must exist that permit such a rapid response. There are two main mechanisms to achieve such a rapid initial increase in glycerol concentration: changes in carbon metabolism and changes in glycerol transport.
Metabolic adjustments:
Adaptation to osmotic stress requires direct metabolic adjustments. Cells must redirect carbon resources toward enhanced production of glycerol, and thus there is significant modulation of central carbon metabolism during osmo-adaptation. There are indications that regulation of glycolysis is crucial for osmotic adaptation; for example, cells deficient in glycerol synthesis are highly osmosensitive. The control of glycolysis and glycerol production appears to be distributed among several enzymes through allosteric control by different metabolites (Hohmann et al. 2007). However, there is direct evidence indicating that the activity of the 6-phosphofructo-2-kinase, Pfk2, which is responsible for controlling the levels of fructose-2,6-bisphosphate (F2,6BP), a key activator of glycolysis, is regulated by the Hog1 MAPK (Dihazi et al. 2004). Therefore, Hog1 may directly control the metabolic flux in response to stress. Along the same lines, recent studies using aerobic, glucose-limited cultures suggest that metabolic regulation rather than de novo enzyme synthesis dominates the initial phase of the adaptive process, at least in the presence of moderately high osmolarity (1 M sorbitol) (Bouwman et al. 2011). Therefore, the regulation of metabolic flux is an important component in Hog1-regulated glycerol accumulation.
Glycerol transport:
Because the lipid bilayer has low permeability for glycerol, specific channel proteins mediate the rapid import and export of glycerol. As a consequence, the control of import and export rates is one mechanism by which the glycerol content inside of the cell can be altered. Thus, the control of the flux of glycerol through the membrane is another key factor for the initial accumulation of glycerol upon osmostress. Stl1, a sugar transporter-like protein whose expression is strongly induced by Hog1 upon stress, might contribute to glycerol accumulation by importing glycerol from the environment in response to stress. However, the fastest mechanism to alter glycerol concentration is via Fps1-mediated glycerol export (Tamás et al. 1999). Fps1 is a member of the aquaporin family of transmembrane channels, and cells that express Fps1 mutant proteins that are constitutively open do not accumulate glycerol and grow poorly in the presence of high osmolarity (Hohmann et al. 2007). In response to osmostress, the Fps1 channel closes to maintain internal glycerol, but this effect seems to be independent of Hog1 (Tamás et al. 1999). On the other hand, direct regulation of Fps1 transport capacity and protein stability by Hog1 has been described for arsenite transport and in response to weak acid treatment (Thorsen et al. 2006; Mollapour and Piper 2007; Beese et al. 2009). In addition, the stress-induced phosphorylation of Rgc2, a novel regulator of Fps1 channel activity, is also partially controlled by the Hog1 MAPK (Mollapour and Piper 2007; Beese et al. 2009). The precise mechanism by which Fps1 is controlled upon osmostress remains unclear.
The combined data indicate that the accumulation of glycerol is a key adaptive response to high osmolarity that is modulated by several mechanisms with different kinetics and different quantitative contributions to achieve proper adaptation to osmostress.
General stress responses
In addition to glycerol, a number of other organic osmolytes, including trehalose, protect yeast from osmostress, not only by counteracting water efflux and reestablishing osmotic balance, but also by playing unique roles in antioxidation, detoxification, and the stabilization of cellular proteins and structures (Mager and Varela 1993; Yancey 2005). Notably, a number of genes that are upregulated by osmostress have similar protective functions as these osmolytes (de Nadal and Posas 2010; Martínez-Montañés et al. 2010). For example, in response to osmostress, a number of genes that protect cells from oxidative damage are upregulated, including genes involved in redox metabolism, mitochondrial function, and the biosynthesis of antioxidative compounds (e.g., TRX2, CTT1, GRE3, and SOD2). Genes that encode the chaperones (e.g., HSP12, HSP104, and HSP42) that protect cells from damage by protein denaturation are also upregulated. It is worth noting that Hog1 has also been implicated in ER stress protection, which is induced in response to the accumulation of unfolded proteins (Bicknell et al. 2010; Torres-Quiroz et al. 2010; Eraso et al. 2011), and in the control of mitophagy, the specific autophagic elimination of mitochondria (Aoki et al. 2011; Mao et al. 2011).
One role of the transcriptional response to a specific stress is to generate a cross-protection to other types of stresses. Osmostress induces many genes that are considered to be part of general stress responses. Conversely, when cells are subjected to a mild stress (e.g., oxidative stress or heat stress), stress response element (STRE)-mediated responses are induced even in the absence of Hog1 (Berry and Gasch 2008). Thus, at 37°, hog1Δ cells can survive on moderate osmostress, such as 0.8 M sorbitol, better than at 30° (Siderius et al. 2000). This protection is not sufficient for hog1Δ cells to survive higher levels of osmolarity.
Regulation of gene expression by osmostress
Global analysis of gene expression upon osmostress:
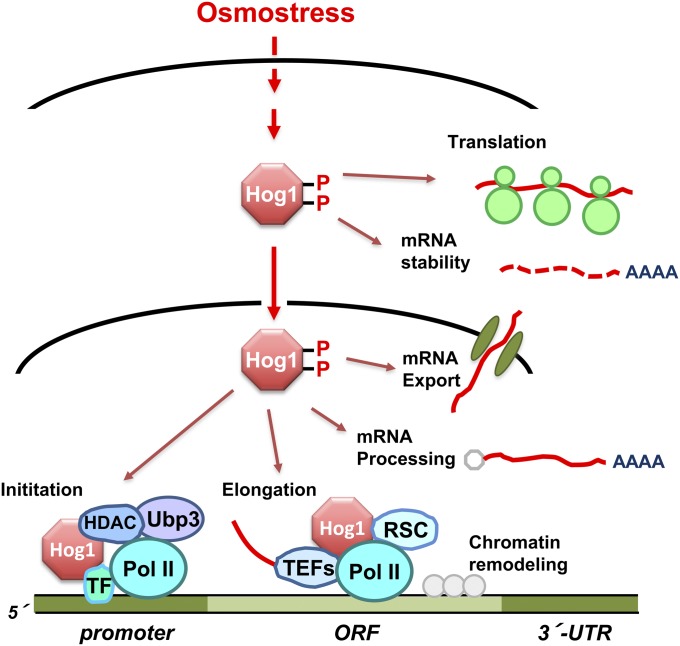
Exposure of yeast to high osmolarity results in profound changes in the physiology of the cell and has a major impact on the capacity of the cell for gene expression. Analysis of the transcriptional changes mediated by Hog1 in response to osmostress may lead to a general understanding of how cells rapidly, precisely, and extremely efficiently adjust the full complement of a transcriptional program in response to extracellular stimuli. Indeed, the Hog1 MAPK plays a key role in the regulation of mRNA biogenesis by controlling several steps in the transcription process (Figure 9) (Hohmann 2002b; de Nadal and Posas 2010; Martínez-Montañés et al. 2010; de Nadal et al. 2011). Although the role of Hog1-dependent gene expression in osmo-adaptation is still incompletely understood, it is clear that long-term adaptation to high osmolarity requires regulated transcription, as a number of mutants in the transcriptional machinery render cells osmosensitive (de Nadal et al. 2004; Zapater et al. 2007; Mas et al. 2009). On the other hand, it has been shown that a membrane-tethered Hog1 construct abolishes short-term transcription responses at certain osmolarities (so that it cannot enter the nucleus). Nevertheless, this Hog1 construct is still able to suppress the osmosensitivity of a hog1Δ strain (Westfall et al. 2008). Therefore, cytoplasmic events caused by the rapid and transient activation of the Hog1 MAPK in response to osmostress—such as the control of glycerol production by direct modulation of metabolic enzymes (Dihazi et al. 2004; Bouwman et al. 2011) and the altered mRNA stability (Molin et al. 2009; Romero-Santacreu et al. 2009; Miller et al. 2011)—might be sufficient for the maintenance of osmotic balance under these experimental conditions without invoking induced gene expression in the nucleus.
Figure 9 .
Control of mRNA biogenesis by the Hog1 MAPK. Once activated upon osmostress, Hog1 controls many aspects of mRNA biogenesis both in the nucleus and in the cytoplasm. Hog1 phosphorylates and activates transcription factors (TFs). Remarkably, Hog1 associates to loci of stress-responsive genes to modulate both initiation and elongation. Hog1 also seems to control mRNA processing, nuclear export, translation and mRNA stability.
Global transcriptional responses to diverse stresses in S. cerevisiae have been studied in detail using gene expression profiling. There are a large number of genes whose transcription is induced in response to osmostress; of these genes, there is one subset of genes that specifically responds to osmostress, whereas another subset of genes responds indiscriminately to diverse types of stresses. Induction of the latter group of genes is known as the environmental stress response (ESR). The ESR consists of ∼300 to ∼600 genes whose expression is upregulated or downregulated by stresses such as DNA damage, heat shock, osmostress, or oxidative stress (Gasch et al. 2000; Causton et al. 2001; Capaldi et al. 2008). The extent and kinetics of the ESR appear to be dependent on the severity of the stress, since cells exposed to increasing stress often display broader changes in gene expression. This general stress response has been implicated in the phenomenon of cross-protection, whereby exposure to a nonlethal dose of one stress can protect cells against unrelated stresses (Berry and Gasch 2008). The genes upregulated by the ESR include genes involved in carbohydrate metabolism, protein metabolism, intracellular signaling, and defense against reactive oxygen species and DNA damage. On the other hand, most of the genes downregulated by the ESR are involved in protein synthesis and in growth-related processes (Gasch 2007; Martínez-Montañés et al. 2010).
It has been clearly established that the stress-responsive MAPKs, such as the mammalian JNK and p38 MAPKs, have a key role in the regulation of transcription upon a diverse array of stresses. In addition to the genes commonly regulated by the ESR, other genes play specific roles in adaptation to particular stresses, and these are also, in varying degrees, under the control of stress-responsive MAPKs. In S. cerevisiae, in which the ESR is not governed by a single regulatory system but by different signaling pathways and transcription factors depending on the type of stress, the Hog1 MAPK is critical for the regulation of ESR genes under osmostress (Posas et al. 2000). Indeed, studies using a hog1Δ mutant strain revealed that, although it depends on the severity of the stress, ∼80% of the genes that are induced upon osmostress depend on the Hog1 MAPK for full induction (Posas et al. 2000; Rep et al. 2000; O’Rourke and Herskowitz 2004; Capaldi et al. 2008).
Hog1 controls gene expression by regulating transcription factors:
One of the well-characterized functions of the family of stress-responsive MAPKs including Hog1 is the regulation of gene expression at the initiation step of transcription. Indeed, Hog1 regulates several unrelated transcription factors, each of which is responsible for controlling the expression of a subset of osmoresponsive genes, either directly or in collaboration with other factors (Molin et al. 2009; Romero-Santacreu et al. 2009; Miller et al. 2011). These Hog1-regulated factors include the transcription activators Hot1, Smp1, Msn1, Msn2, and Msn4 and the transcription repressor Sko1 (de Nadal and Posas 2008). These factors can act independently or in combination at specific promoters to elaborate a dynamic transcriptional response to stress (Ni et al. 2009). A recent study that combined dynamic transcriptome analyses with an analysis of mRNA synthesis rates suggested that additional transcription factors could exist that act in association with these factors and are important for the response to osmostress (Molin et al. 2009; Romero-Santacreu et al. 2009; Miller et al. 2011). Therefore, these factors could also be substrates of Hog1. Overall, it is clear that a collaborative effort of many different transcription factors is needed for gene induction upon osmostress.
Direct phosphorylation of promoter-specific transcription factors is the best-understood mechanism by which the Hog1 MAPK modulates initiation of transcription. Examples of regulation by direct phosphorylation have been reported for the MEF2-like activator Smp1 and the ATF/CREB-family member Sko1 (Nehlin et al. 1992; Vincent and Struhl 1992). In vivo coprecipitation and phosphorylation studies showed that Smp1 and Sko1 interact with, and are directly phosphorylated by, Hog1 (Proft et al. 2001; de Nadal et al. 2003). Regulation of Sko1 function has an extra layer of complexity: while Sko1 acts as a transcription activator in the presence of stress, it acts as a transcriptional repressor in the absence of stress. In the absence of stress, Sko1 represses stress-inducible genes by recruiting the general transcription corepressor complex Ssn6 (Cyc8)–Tup1 to their promoters (Garcia-Gimeno and Struhl 2000; Pascual-Ahuir et al. 2001). Induction of Sko1-dependent genes requires the release of this repression, and this process is completely dependent on Hog1 (Pascual-Ahuir et al. 2001; Proft and Struhl 2002). In fact, Hog1-dependent phosphorylation of Sko1 changes Sko1 from a repressor into an activator by modifying its association with the Tup1–Ssn6 complex and allows the recruitment of the chromatin-remodeling complexes SAGA and SWI/SNF to osmostress-inducible promoters (Rep et al. 2001; Proft and Struhl 2002; Guha et al. 2007; Kobayashi et al. 2008). These examples illustrate that the direct phosphorylation of transcription factors by activated Hog1 is a key regulatory element for induction of gene expression in response to osmostress.
Hog1 controls gene expression by associating with chromatin:
Hog1 also affects the functions of transcription factors by mechanisms other than by direct phosphorylation. Indeed, phosphorylation by Hog1 may not be critical for regulation of a number of transcriptional regulators that are under Hog1 control, such as Msn2, Msn4, and Hot1 (Alepuz et al. 2003). Msn2 and Msn4 mostly control induction of ESR genes through the STRE (Martínez-Pastor et al. 1996; Schmitt and McEntee 1996), and Hot1 affects expression of a small subset of Hog1-dependent genes, including GPD1 and GPP2, which are involved in glycerol biosynthesis, as well as STL1, which encodes a glycerol/proton symporter (Rep et al. 1999b, 2000; Ferreira et al. 2005).
The fact that the nuclear retention of Hog1 upon osmostress is dependent on the presence of the transcription factors that are downstream of Hog1 suggested that these factors could act as nuclear anchors for Hog1 by engaging in stable interactions with it (Reiser et al. 1999; Rep et al. 1999b). Indeed, Hog1 does associate with chromatin, and it does so via physical interactions with transcription factors. For example, recruitment of Hog1 to the CTT1 promoter requires the transcription factors Msn2 and Msn4, whereas recruitment of Hog1 to the STL1 promoter depends on the transcription activator Hot1 (Alepuz et al. 2001). It is worth noting that Hog1 binds only to osmo-responsive genes (Pascual-Ahuir et al. 2006; Pokholok et al. 2006; Proft et al. 2006). An accumulation of Hog1 in the nucleus is not sufficient for its association with chromatin, since addition of a nuclear localization signal to Hog1 does not result in its enhanced chromatin association. However, binding of Hog1 to chromatin does depend on its catalytic activity (Alepuz et al. 2001). Thus, in contrast to the more traditional scenario in which a MAPK controls transcription only indirectly by phosphorylating transcription factors, the persistent presence of Hog1 at target promoters clearly indicates that Hog1 itself plays an important role in the regulation of transcription initiation (Alepuz et al. 2001; Chellappan 2001; Proft and Struhl 2002). Systematic genome-wide analyses of the binding of transcription factors and of Hog1 to chromatin, combined with gene expression profiling, have shown that, in response to osmostress, Hog1 can specifically regulate, and integrate, the stress responses that occur at different promoters. This effect is accomplished by Hog1 via modulation of the individual contribution of transcription factors, such as Msn2/Msn4, Sko1, and Hot1, in a promoter-specific context that results in a complex and highly specific control of transcriptional networks (Proft et al. 2005; Capaldi et al. 2008; Ni et al. 2009).
Other yeast MAP kinases such as Fus3, Kss1, and Mpk1 are also recruited to chromatin (Pokholok et al. 2006; Kim et al. 2008). Furthermore, structurally and functionally unrelated yeast signaling kinases, including Snf1 (Lo et al. 2005; Li et al. 2006; Pokholok et al. 2006), have been reported to be recruited to chromatin. Similar binding of signaling kinases to chromatin, implying their direct roles in gene regulation, has now been shown to occur in several other organisms including mammals and Drosophila (Chow and Davis 2006; Edmunds and Mahadevan 2006; de Nadal and Posas 2010; de Nadal et al. 2011).
Transcription initiation at osmostress-responsive promoters:
The observation that Hog1 kinase activity is needed for transcriptional activation, even though phosphorylation of transcription factors is not an absolute requirement for transcription initiation, indicates that Hog1 can induce activation of gene expression by a mechanism other than phosphorylation of transcription activators. Indeed, recruitment of the RNA Pol II machinery to osmoresponsive genes is dependent on both activated Hog1 and the presence of specific transcription factors. The facts that Hog1 tightly associates with the largest subunit of RNA Pol II and that the artificial tethering of Hog1 to chromatin is sufficient to induce gene expression upon osmostress suggest that Hog1 serves to recruit the basic transcriptional machinery to stress-responsive promoters (Alepuz et al. 2003).
It has been shown that the extent of transcriptional activation is regulated by the Ubp3 ubiquitin protease. Ubp3 is targeted to stress-responsive genes by Hog1, and its activity is regulated by direct phosphorylation by Hog1. Thus, the regulation of the turnover of specific transcription factors and/or RNA PolII at the promoter seems to be important for the dynamics of gene expression upon stress (Solé et al. 2011). Furthermore, genetic and biochemical data suggests that, in addition to binding to transcriptional activators and facilitating RNA Pol II recruitment, Hog1 is also important for the recruitment of basic transcription complexes such as SAGA, Mediator, and SWI/SNF to osmo-responsive promoters. Several observations indicate that, whereas Mediator is crucial for proper gene induction under both mild and severe osmostress conditions, the role of SAGA is dependent on the strength of the osmostress. Thus, the requirement for a given transcriptional complex to regulate a promoter might depend on the severity of osmostress and be determined through the regulation of interactions among transcriptional complexes (Zapater et al. 2007). The recruitment of the SWI/SNF chromatin-remodeling complex to promoters also depends on the presence of Hog1. Although elimination of components of the SWI/SNF complex does not lead to clearly observable effects on transcription, modification of chromatin might still be important for efficient transcription in response to osmostress (Proft and Struhl 2002).
Although histone deacetylation has been classically associated with repression of gene expression (Robyr et al. 2002), there are many genes for which histone deacetylation is associated with transcription induction (Bernstein et al. 2000; Shahbazian and Grunstein 2007). In fact, the Rpd3 histone deacetylase (HDAC) complex plays an important role in induction of gene expression by osmostress. Rpd3 belongs to a five-member family of related histone deacetylases, and it has been reported to regulate the expression of a large number of genes (Yang and Seto 2008). There are two different Rpd3-containing HDAC complexes, the larger Rpd3L and the smaller Rpd3S, that share a common core composed of Rpd3, Sin3, and Ume1. Whereas the Rpd3L complex is recruited to promoters to enhance transcription initiation, the Rpd3S complex controls promoter fidelity by suppressing spurious intragenic transcription during elongation (Carrozza et al. 2005; Keogh et al. 2005; Li et al. 2007b,c; Biswas et al. 2008). Cells defective in Rpd3 and in other components of the Rpd3L complex are osmosensitive and show compromised expression of osmostress-responsive genes controlled by Hog1. Hog1 binds to an Rpd3 complex (presumably Rpd3L) and, upon stress, recruits it to specific osmostress-responsive genes. Binding of the Rpd3 complex to specific promoters leads to histone deacetylation, entry of RNA polymerase II, and induction of gene expression (de Nadal et al. 2004). It should be added that the role of the Rpd3 complex at osmostress-responsive promoters need not be restricted to alteration of chromatin structure, but it might also provide a unique binding surface or recognition motifs for the recruitment of transcription activators.
Transcription elongation of osmostress-responsive genes:
Elongation is also a critical phase of transcription that is highly regulated, and modification of the RNA Pol II carboxy-terminal domain is just one example of such regulation (Saunders et al. 2006; Egloff and Murphy 2008; Fuda et al. 2009). Upon osmostress, the Hog1 MAPK interacts with RNA Pol II as well as with the general components of the transcription elongation complex while these are engaged in elongation (Proft et al. 2006). In addition to its association with the promoter regions of osmostress-responsive genes, Hog1 is also present on the coding regions of these genes, and it travels with elongating RNA Pol II (Pascual-Ahuir et al. 2006; Pokholok et al. 2006; Proft et al. 2006). It should be noted that the binding of Hog1 to the coding regions is independent of promoter-bound transcription factors, but is dependent on the 3′-UTR region of osmostress-responsive genes. The mechanism by which Hog1 is recruited to the 3′-regions of osmostress-responsive genes is unclear. By fusing a Hog1-independent promoter to the coding region of a Hog1-dependent gene, it is possible to uncouple Hog1-dependent transcription initiation from transcription elongation. Thus, it has been demonstrated that the presence of Hog1 at coding regions is essential for increased association of RNA Pol II with the coding region, suggesting that Hog1 directly affects the process of elongation (Proft et al. 2006). Other yeast signaling kinases, such as Fus3 or PKA, also associate with the coding regions of activated genes (Pokholok et al. 2006), which indicates that signaling kinases play a role in transcription beyond initiation.
Remodeling of chromatin in response to osmostress:
The packaging of DNA into nucleosomes affects all phases of the transcription cycle from the binding of activators and formation of a pre-initiation complex to elongation. Thus, nucleosome positioning and dynamics is another layer of transcription regulation (Cairns 2009; Jiang and Pugh 2009). As in the case of initiation, transcription elongation is also affected by chromatin structure, which is regulated by several protein factors that covalently modify histones or temporarily remove, disassemble, and reassemble nucleosomes (Li et al. 2007a). Chromatin-remodeling complexes utilize the energy of ATP hydrolysis to alter histone-DNA contacts by transiently unwrapping DNA, forming DNA loops, sliding nucleosomes, completely displacing the histones from DNA, or replacing histone subunits. Transcriptional responses to stress and chromatin structure alterations are tightly linked (Shivaswamy and Iyer 2008).
In response to osmostress, the nucleosome organizations of the osmostress-responsive genes undergo a dramatic change that depends on Hog1 and on the RSC chromatin-remodeling complex. The RSC complex (including Rsc1, Rsc2, Rsc3, etc.) is a distinct member of the SWI/SNF family and is known to modify nucleosome structure. Upon osmostress, the Hog1 MAPK physically interacts with RSC to direct its association with the coding region of osmostress-responsive genes, suggesting that this activity could be a major role of Hog1 during elongation. Notably, in RSC mutants, RNA Pol II still accumulates at the promoter regions of the osmostress-responsive genes, but not at their coding regions, implying that elongation is specifically suppressed. Furthermore, RSC mutants display reduced expression of osmostress-responsive genes and enhanced osmostress sensitivity (Mas et al. 2009). Cell adaptation under acute osmostress might thus depend on a burst of transcriptional activity that can occur only with efficient nucleosome eviction. Remarkably, the exposure of cells to mild osmostress results in bimodal expression of osmostress-responsive genes: expression levels among a population of equally stimulated cells are not continuously distributed, but display low and high peaks (Pelet et al. 2011). This bimodality arises at the transcriptional level: even if Hog1 is activated to a similar level in all cells, the transcriptional outcome is determined by a slow stochastic transition from a repressed transcriptional state to an activated state. This transition seems to depend on chromatin structure.
In addition to the RSC complex, two other chromatin remodelers have been found to associate with stress-responsive genes; the SWI/SNF complex, which has been discussed above, and the INO80 complex, which contains subunits such as Ino80 and Arp8. Disruption of the INO80 complex-specific gene ARP8 results in extended expression of, and a delay in nucleosome reassembly at, stress-responsive genes during osmostress adaptation (Klopf et al. 2009). Therefore, chromatin-remodeling complexes and chromatin-modifying enzymes are key elements for stress-mediated gene expression, and a dynamic balance among different chromatin-remodeling complexes seems to be required for proper regulation of stress-responsive genes.
Control of mRNA processing and stability by Hog1:
Eukaryotic mRNAs are synthesized as precursors by RNA Pol II and are subsequently extensively modified, spliced, cleaved at the 3′-end, and polyadenylated. In addition, nuclear export and translation of mRNAs is coordinated differentially. At present, it is unclear whether osmostress-responsive genes are post-transcriptionally regulated by specific mRNA-binding proteins and what potential role the Hog1 MAPK might play in their regulation. In mammalian cells, stress-responsive p38 MAPK contributes to stabilization of cytokine/stress-inducible mRNAs, through an ARE (AU-rich elements present in 3′-UTR)-targeted mechanism. AREs regulate mRNA turnover by modulating poly(A)-shortening rates and the subsequent decay of mRNA. In S. cerevisiae, inhibition of the Hog1 pathway by the p38 inhibitor SB202190 leads to destabilization of ARE-bearing transcripts, suggesting that a similar mechanism exists in yeast (Vasudevan and Peltz 2001). Although this is a very interesting mechanism by which Hog1 might influence gene expression, its role in osmostress responses is unknown.
Genome-wide analyses have shown that there is a clear and specific regulation of stress-responsive mRNA in comparison with global mRNAs (Molin et al. 2009; Romero-Santacreu et al. 2009; Miller et al. 2011). For example, under mild osmostress, destablization of a broad range of mRNAs is induced, whereas osmostress-inducible mRNA synthesis is upregulated and the half-life of these mRNAs is extended. In a hog1Δ mutant, mild osmostress induces global stabilization of mRNA and P-body formation (Romero-Santacreu et al. 2009). Notably, stress-responsive mRNAs are selectively stabilized or degraded, depending on the phase of the response to stress, namely, initial shock, induction, or recovery (Miller et al. 2011). It is clear from these reports that Hog1 has an effect on mRNA stability, especially for osmostress upregulated genes. However, the mechanism by which Hog1 controls the stability of mRNAs is unclear.
Regulation of cell-cycle progression by osmostress
In S. cerevisiae, a highly regulated and complex network of proteins governs cell-cycle progression, although major events are controlled by a single cyclin-dependent kinase (CDK) Cdc28. The activity of Cdc28 is regulated mainly through the synthesis and degradation of various cyclins and inhibitors that bind to Cdc28 (Clotet and Posas 2007). As the presence of various stresses such as heat stress, DNA damage, and extracellular hyperosmolarity critically affects progression through the cell cycle, cells must modulate the cell cycle to allow for proper cellular adaptation (Flattery-O’Brien and Dawes 1998; Li and Cai 1999; Wang et al. 2000; Alexander et al. 2001). For cells under these stress conditions, controlled delay of cell-cycle progression is very important, since it enables cells to adapt to the new environmental conditions before moving through vulnerable cell-cycle transition periods.
As environmental stresses can occur at any cell-cycle stage, in principle, all phases of the cell cycle must be regulated by stress-activated mechanisms. In mammals, the p38 stress-responsive MAPK has been implicated in regulating G1 phase, S phase, as well as a G2/M checkpoint, in response to several stimuli, including osmostress (Dmitrieva et al. 2002; Joaquin et al. 2012). In yeast, the Hog1 MAPK induces a rapid and transient delay at various stages of the cell cycle to permit the full development of adaptive responses before cell-cycle progression resumes (Figure 10A) (Clotet and Posas 2007; Yaakov et al. 2009).
Figure 10 .
Control of the cell-cycle progression by the Hog1 MAPK. (A) The dominant species of the cyclin/Cdc28 complex at each cell-cycle phase are shown around the circle that represents the cell cycle (G1→S→G2→M). Once activated by osmostress, Hog1 seems to modulate all phases of the cell cycle. In the G1 and G2 phases, Hog1 controls cell-cycle regulators both directly and indirectly, and Hog1 also regulates expression of cyclins. Hog1 also modulates the S and M phases, but the mechanisms remain unclear (not shown). (B) Details of the control of the G1/S transition by Hog1. The transition from G1 to S phase is mediated by the expression of cyclins Cln1,2 and Clb5,6, and their binding to the Cdc28 kinase. Initially, Clb5,6/Cdc28 is inhibited by the Sic1 (CDKi). As the activity of Cln1,2/Cdc28 increases, Sic1 is phosphorylated at multiple sites, prompting ubiquitination of Sic1 by the SCF (Cdc4) complex, and its degradation by proteasome. This degradation of Sic1 releases active Clb5,6/Cdc28, which then promotes DNA replication. Osmostress-activated Hog1 delays G1/S transition both by inhibiting transcription of cyclin genes (both CLN and CLB), and by directly phosphorylating Sic1 at Thr-173, which inhibits ubiquitination of Sic1 and stabilizes Sic1.
G1/S transition:
In yeast, the G1/S transition is controlled by the interplay of several cyclins. At the beginning of G1, the Cln3 cyclin is sequestered in the cytoplasm by the Whi3 retention factor (Bellí et al. 2001; Garí et al. 2001). In the late G1 phase, nuclear accumulation of Cln3 triggers the phosphorylation of the transcription repressor Whi5 by Cln3-bound Cdc28 (Cln3/Cdc28) (Costanzo et al. 2004; de Bruin et al. 2004). Phosphorylated Whi5 dissociates from the transcription complexes SBF and MBF, which leads to the transcription of a second wave of cyclins (Cln1, Cln2, Clb5, and Clb6). The G1 cyclins Cln1 and Cln2 are functionally redundant and are abbreviated as Cln1,2; similarly, the B-type cyclins Clb5 and Clb6 are redundant and are abbreviated as Clb5,6. The activity of Cln1,2/Cdc28 stimulates bud formation and phosphorylation of the CDK inhibitor Sic1 (Figure 10B). Unphosphorylated Sic1 binds and inhibits Clb5,6/Cdc28. When several residues in Sic1 are phosphorylated by Cln1,2/Cdc28, Sic1 is poly-ubiquitinated and degraded by the proteasome (Verma et al. 1997). Sic1 degradation removed Sic1 inhibition of Clb5,6/Cdc28. Activated Clb5,6/Cdc28 can phosphorylate Sic1 at the same residues as those phosphorylated by Cln1,2/Cdc28. Thus, Sic1 degradation accelerates by the positive feedback loop, resulting in an abrupt rise in Clb5,6/Cdc28 activity, which drives cells into S phase. DNA replication is initiated when Clb5,6/Cdc28 phosphorylates the replication proteins Sld2 and Sld3, which are components of the pre-initiation complex (Masumoto et al. 2002; Tanaka et al. 2007). Therefore, at the end of G1, the activity of Clb5,6/Cdc28 depends on both the levels of the Clb5,6 cyclins and the levels of the inhibitor Sic1 (Schwob et al. 1994; Verma et al. 1997; Cross et al. 2007).
When yeast cells are exposed to high osmolarity (e.g., 0.4 M NaCl), the Hog1 MAPK is transiently activated for ∼30 min, and a corresponding cell-cycle delay in G1 is observed (Bellí et al. 2001). That this delay is caused by activated Hog1, and not by other effects of osmostress, can be demonstrated using genetic means to activate Hog1 in the absence of osmostress. Experimentally, this can be achieved by high-temperature inactivation of an sln1-ts mutant or expression of the constitutive PBS2DD allele, both of which are upstream of the Hog1 activation pathway. If Hog1 activity is sustained for an extended period, cells undergo a programmed cell death that requires the action of the nuclear serine proteinase Nma111 and the SCFCDC4 ubiquitin ligase (Vendrell et al. 2011). However, a shorter activation of the HOG pathway results in cell-cycle delay in G1. Hog1 controls the G1/S transition by acting on two different components of the basic cell-cycle machinery (Escoté et al. 2004; Clotet and Posas 2007; Zapater et al. 2007 Adrover et al. 2011). First, Hog1-mediated G1 arrest is partially mediated by downregulation of expression of the G1 cyclins Cln1,2 and of the S-cyclin Clb5 (Bellí et al. 2001). The exact nature of the mechanism that delays the expression of these SBF/MBF-dependent genes under osmotic stress remains unknown. Second, Hog1 promotes a cell-cycle delay in G1 by direct phosphorylation of Sic1 at a specific residue (Escoté et al. 2004). When Cln1,2/Cdc28 activities reach a threshold level, Sic1 is phosphorylated, then ubiquitinated by the Cdc4 ubiquitin ligase, and eventually degraded by the proteasome. If Hog1 is activated by osmostress, it interacts physically with Sic1 and phosphorylates another residue (Thr-173) at the carboxyl terminus of Sic1. This phosphorylation inhibits Sic1 ubiquitination (Escoté et al. 2004; Zapater et al. 2005). Thus, Sic1 degradation is inhibited, resulting in prolonged inhibition of Clb5,6/Cdc28 and a delay in the G1/S transition. Incidentally, it should be noted that the same Thr-173 in Sic1 is targeted by the TOR pathway to delay cell-cycle progression in G1 (Zinzalla et al. 2007).
Mathematical modeling and quantitative in vivo experiments have defined the differential roles of Hog1 in G1 delay (Adrover et al. 2011). There are distinct effects of Hog1 at different cell-cycle stages in response to osmostress: early in G1, it inhibits cyclin expression, and later in G1, it inhibits Sic1 degradation. Of the three cyclin genes whose transcription is inhibited by Hog1, inhibition of CLN1,2 expression delays bud morphogenesis, and inhibition of CLB5 expression delays DNA replication and entry into S phase. Later in G1, when these cyclins are already expressed, inhibition of cyclin expression can no longer prevent cell-cycle progression. Instead, Hog1-mediated phosphorylation and inhibition of the degradation of Sic1 prevents active Clb5/Cdc28 from initiating DNA replication. Thus, these two distinct mechanisms that operate at different time points ensure that no premature entry into S phase occurs under osmostress conditions.
S phase:
The Hog1 MAPK is also able to modulate S-phase progression in response to osmostress. During S phase, replication of the genome occurs, which is a highly ordered process involving many proteins. The assembly of the replication complex (RC) at origins of replication begins with the formation of the pre-replicative complex (pre-RC) during late mitosis to G1. In the S phase, the pre-RC is converted into a fully assembled pre-initiation complex. Further conversion to a fully functional RC is accompanied by various molecular events, including phosphorylation of the Dpb2 subunit of the DNA polymerase by CDK. These processes are dependent on the activities of S-phase CDK (Clb5,6/Cdc28) and Dbf4-dependent kinase (the Cdc7/Dbf4 complex). When cells are stressed in early S phase, Hog1 controls the S phase by delaying the expression of the S-phase cyclins Clb5,6 (see G1/S transition). If cells are stressed later in S phase, Hog1 interacts with components of the replication complex and delays phosphorylation of the Dpb2 subunit of the DNA polymerase (Yaakov et al. 2009). These effects of Hog1 are independent of the S-phase DNA checkpoint or of the known Hog1 targets Sic1 and Swe1.
One possible reason to delay the cell cycle when osmostress occurs in the S phase is the need to prevent replication from interfering with the necessary transcription of adaptive genes. Adaptive responses to osmostress require that expression of a very large number of genes is induced. It is therefore conceivable that initiating or ongoing replication might occur on the genes that are being transcribed for adaptation. It is easy to see that if the large replication complex and the transcription complex attempt to occupy the same space, they would interfere with each other’s function. Furthermore, it has been shown that a collision between RNA Pol II and DNA polymerase leads to transcription-associated recombination (Aguilera 2002, 2005; Aguilera and Gómez-González 2008). Thus, delaying replication in response to osmostress must be important both to provide proper adaptive gene expression and to prevent genomic instability.
G2 phase:
Cell-cycle progression from the S into the G2 phase depends mainly on another wave of cyclin production: the mitotic cyclins Clb1 and Clb2. The Mcm1/SFF (Mcm1/Fkh2/Ndd1) complex is the transcription factor that regulates expression of CLB1 and CLB2 (Althoefer et al. 1995; Maher et al. 1995; Jorgensen and Tyers 2000). Further cell-cycle progression from G2 into mitosis is controlled by the morphogenetic checkpoint. The G2/M transition depends on the activity of CDK that is associated with either of these mitotic cyclins (Clb1,2/Cdc28). The activity of the Clb2/Cdc28 complex is inhibited by phosphorylation at a conserved tyrosine in Cdc28 by the kinase Swe1 (the ortholog of Schizosaccharomyces pombe and mammalian Wee1) and is reactivated by the phosphatase Mih1 (the ortholog of S. pombe and mammalian Cdc25). When the formation of the septin ring is completed on the neck between mother and daughter cells, the morphogenetic checkpoint recruits a complex of Hsl1 (a septin-dependent protein kinase) and Hsl7 (a protein that binds to Swe1) to Swe1, which targets Swe1 for destruction. Swe1 does not affect cell-cycle progression under unstressed, exponentially growing conditions (Amon et al. 1992). However, when bud formation is impaired by various stresses, Swe1 remains active, inactivates the mitotic CDK, and delays the cell cycle (McMillan et al. 1999). Perturbations of the actin cytoskeleton, rather than the bud size itself, seem to stabilize Swe1 (McNulty and Lew 2005).
Activation of Hog1 upon osmostress induces a cell-cycle delay in G2 by decreasing Clb2/Cdc28 activity and by downregulating CLB2 transcription (Alexander et al. 2001; Clotet et al. 2006). The mechanisms that Hog1 uses to downregulate CLB2 transcription are not known, but this downregulation could be a secondary effect due to the Hog1-induced decrease in Clb2/Cdc28 activity. In contrast, the mechanism by which Hog1 decreases Clb2/Cdc28 activity is clearly a result of Hog1 acting on the machinery of the morphogenetic checkpoint that controls Swe1 levels. Thus, activated Hog1 interacts with and directly phosphorylates Hsl1 at a residue within the Hsl7-docking site. This Hsl1 phosphorylation promotes the delocalization of Hsl7 from the neck, resulting in Swe1 accumulation and G2 arrest (Clotet et al. 2006). In mutant cells that contain a nonphosphorylatable Hsl1, Hog1 activity cannot promote Hsl7 delocalization, fails to accumulate Swe1, and fails to arrest at G2. This explains why the mitogenic checkpoint is sensitive to osmotic stress.
Exit from mitosis:
Exit from mitosis after chromosome segregation is controlled by a signaling cascade termed the Mitotic Exit Network (MEN). Activation of MEN is initiated by the activation of Tem1, a G-protein that is located in the spindle pole body (Morgan 1999). When cells undergo anaphase, the spindle pole body enters into the daughter cell where the GEF for Tem1, Lte1, is localized (Pereira et al. 2000). Activated (GTP-bound) Tem1 then binds to and activates the Cdc15 kinase, a critical component of MEN, which leads to activation of the phosphatase Cdc14. The Cdc14 protein phosphatase is tightly regulated by a competitive inhibitor Net1, which holds Cdc14 in an inactive state in the nucleolus during most of the cell cycle except during anaphase and telophase. Cdc14 is released by MEN or by the FEAR (Cdc Fourteen Early Anaphase Release) network, and spreads throughout the nucleus and cytoplasm to induce exit from mitosis. Cdc14 activates the Anaphase Promoting Complex (APC)/Cdh1, which promotes ubiquitination and degradation of the remaining B- type cyclins. Cdc14 also directly dephosphorylates CDK substrates; for example, Cdc14 dephosphorylates and stabilizes Sic1 (Stegmeier and Amon 2004).
Exit from mitosis could also be regulated by the Hog1 MAPK under osmotic stress. In response to osmostress, MEN mutants exit from mitosis in a manner that is dependent on Hog1. In such MEN mutants, the HOG pathway seems to drive exit from mitosis by promoting the function of FEAR network that activates Cdc14, although the exact mechanism remains unclear (Reiser et al. 2006).
Other downstream effectors of the Hog1 MAPK
In addition to phosphorylating components of the transcriptional and cell-cycle machineries, the Hog1 MAPK also phosphorylates other cytoplasmic and nuclear proteins. Recent phospho-proteomic studies identified a number of proteins that are phosphorylated upon osmostress (Soufi et al. 2009). In addition, cells with mutations in kinases and phosphatases that play a role in the HOG pathway showed changes in the phospho-proteome of the cell even under normal osmotic conditions (Bodenmiller et al. 2010). These analyses suggest that a large number of Hog1 substrate proteins must exist that have not been previously characterized. Below, we will discuss several well-defined substrates of Hog1 that are known to have important roles in osmo-adaptation.
Ion channels:
Activation of Hog1 in response to osmostress induces the phosphorylation of at least two proteins located at the plasma membrane, the Nha1 Na+/H+ antiport and the Tok1 potassium channel (Proft and Struhl 2004). Immediately following the start of osmostress, passive water efflux rapidly increases intracellular Na+ concentration, which causes dissociation of proteins from chromatin. Activated Hog1 phosphorylates and thus stimulates Nha1 activity, leading to rapid pumping-out of excessive Na+. This activity is crucial for the rapid and selective re-association of stress-responsive transcription factors with chromatin. Phosphorylation of the Tok1 K+ channel also increases its activity, although its contribution to adaption seems to be less important than that of Nha1. These initial responses to osmostress precede, and prepare for, the activation of stress-response genes that depend on Hog1.
Control of ionic fluxes during long-term adaptation occurs through regulation of the expression of the Na ATPase ENA1. Thus, a single MAP kinase coordinates diverse responses to stress that are temporally, spatially and mechanistically distinct, thereby providing very rapid initial relief, which facilitates subsequent changes in gene expression that permit long-term adaptation to harsh environmental conditions.
Protein kinases regulated by Hog1:
There is a transient decrease in protein synthesis in response to increases in external osmolarity that is caused by a decrease in amino acid uptake, repression of ribosomal protein gene expression, and a decrease in translation efficiency (Norbeck and Blomberg 1998; Uesono and Toh-E 2002). The Hog1 MAPK is not involved in the initial inhibition of translation, but rather in the reactivation of translation under stress, which functions as an adaptation mechanism (Uesono and Toh-E 2002). The cytoplasmic Rck2 kinase, which is structurally homologous to mammalian CaM kinases, is directly phosphorylated and regulated by Hog1 (Bilsland-Marchesan et al. 2000; Teige et al. 2001). Reduction of protein synthesis upon osmostress was similar in hog1Δ and rck2Δ cells, which suggests that the effect of Hog1 on translation is mediated by the Rck2 kinase. Rck2 may affect translation by directly regulating the elongation factor EF-2, but an effect on initiation factors cannot be excluded (Teige et al. 2001). An analysis of polysome-associated mRNAs showed that many genes that are not transcriptionally induced are translated more efficiently under osmostress conditions. A similar analysis of hog1Δ cells showed that the effect of Hog1 on translation was even stronger than the effect on transcription, which highlights the importance of translational control for the fine tuning of adaptive responses (Warringer et al. 2010).
Perspectives
In the second edition of “the Yeast Books” published in mid-1990s, there was only a very brief mention of budding yeast osmoregulation, which occupied no more than half a page (MacNeill and Nurse 1997). The relevant knowledge accumulated in the intervening 15 years, which we have tried to summarize in this review, is nothing less than astounding. Perhaps as a result, many important new questions have become apparent. Below, we have made a somewhat subjective list of questions that are particularly important. Considering the rapid progress made in the past 15 years, we can optimistically expect that many of these questions will be answered by the next edition of the YeastBook.
We now have a clear outline of the upstream signaling in osmostress pathway. However, under closer inspection, many unsolved questions remain. For example, the mechanism by which the Sln1 osmosensor detects changes in osmolarity is unclear. Because the Sln1 histidine kinase is structurally similar to the bacterial osmosensor EnvZ (Tokishita and Mizuno 1994; Yoshida et al. 2007; Wang et al. 2012), it is reasonable to expect that their activation mechanisms are also related. Therefore, a parallel investigation of these two osmosensors, emphasizing both their similarities and differences, might be productive. Another important question is how the Sln1-Ypd1-Ssk1 phosphorelay is regulated, and, in particular, the mechanism by which the stability of Ssk1∼P changes so drastically upon osmostress stimulation.
Although mammals do not have any homolog of the Sln1 osmosensor, they do have mucin-like transmembrane proteins that are structurally similar to the Msb2/Hkr1 osmosensors. Therefore, elucidation of the mechanism by which Msb2/Hkr1 detect osmolarity changes might shed light on the osmosensing mechanism in higher eukaryotes. Furthermore, it needs to be examined whether the Msb2/Hkr1 osmosensors actually regulate the activity of Cdc42, as currently hypothesized. The function of Sho1 in signaling should also be further delineated. Traditionally, this molecule is considered to be a passive membrane anchor for the Pbs2 MAPKK. However, genetic evidence suggests that Sho1 might have a more active role in signaling, perhaps by serving as a platform around which a signaling complex is organized.
The mechanism that controls the crosstalk among the MAPK signaling pathways is another open question. For example, how activation of the Kss1 MAPK by osmostress is prevented in wild-type cells, while this inhibition is abrogated in hog1Δ mutant cells, has been intensely investigated but without any clear answer. Eventually, understanding the global signaling network including the HOG signaling pathways and other intracellular signaling pathways should be an important goal in the next decade.
Regarding downstream effector functions, the number of unsolved questions is commensurate with the breadth of Hog1 functions. There is evidence to suggest that activated Hog1 elicits the production/accumulation of protective osmolytes through transcriptional induction of metabolic enzymes as well as by direct modulation of metabolic flux. Thus, establishment of how Hog1 regulates the metabolic network, both at the transcriptional and posttranscriptional levels, is important for understanding of the basic logic of the cellular response to environmental osmostress. At the metabolic level, it is essential to identify the key enzymes whose activities are directly controlled by Hog1. We must also elucidate the still unclear details of the regulation by osmostress of transcription initiation and elongation, mRNA processing, mRNA stability, nuclear export, and translation.
Osmostress induces cell-cycle delays, which permit cells to adapt to the stress before progressing into vulnerable cell-cycle transitions. Hog1 uses several molecular strategies, alone or in combination, to arrest cells at safer phases in the cell cycle until an osmotic balance is re-established. Some of these mechanisms have become clearer in recent years, but others remain obscure. Because of the advanced knowledge available regarding basic cell-cycle regulation, a model-based simulation will be particularly helpful in investigation of the modulation of the cell cycle by osmostress.
Finally, the search for Hog1 substrates is far from complete. Identification and characterization of novel Hog1 targets will serve to define new Hog1 functions as well as the regulatory mechanisms under its control.
Acknowledgments
We thank Pauline O’Grady and Kazuo Tatebayashi for critical reading of the manuscript. The laboratory of H.S. is supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The laboratory of F.P. is supported by grants from the Ministerio de Economia y Competitividad (Spanish Government), the Consolider Ingenio 2010 Programme, and a FP7 UNICELLSYS grant. F.P. is also supported by the Fundación Marcelino Botín and by the Acadèmia program from Institució Catalana de Recerca i Estudis Avançats (Generalitat de Catalunya).
Footnotes
Communicating editor: P. Pryciak
Literature Cited
- Adrover M., Zi Z., Duch A., Schaber J., González-Novo A., et al. , 2011. Time-dependent quantitative multicomponent control of the G1-S network by the stress-activated protein kinase Hog1 upon osmostress. Sci. Signal. 4: ra63. [DOI] [PubMed] [Google Scholar]
- Aguilera A., 2002. The connection between transcription and genomic instability. EMBO J. 21: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera A., 2005. mRNA processing and genomic instability. Nat. Struct. Mol. Biol. 12: 737–738 [DOI] [PubMed] [Google Scholar]
- Aguilera A., Gómez-González B., 2008. Genome instability: a mechanistic view of its causes and consequences. Nat. Rev. Genet. 9: 204–217 [DOI] [PubMed] [Google Scholar]
- Albertyn J., Hohmann S., Thevelein J. M., Prior B. A., 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 14: 4135–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alepuz P. M., Jovanovic A., Reiser V., Ammerer G., 2001. Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell 7: 767–777 [DOI] [PubMed] [Google Scholar]
- Alepuz P. M., de Nadal E., Zapater M., Ammerer G., Posas F., 2003. Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 22: 2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. R., Tyers M., Perret M., Craig B. M., Fang K. S., et al. , 2001. Regulation of cell cycle progression by Swe1p and Hog1p following hypertonic stress. Mol. Biol. Cell 12: 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoefer H., Schleiffer A., Wassmann K., Nordheim A., Ammerer G., 1995. Mcm1 is required to coordinate G2-specific transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 15: 5917–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A., Surana U., Muroff I., Nasmyth K., 1992. Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. Nature 355: 368–371 [DOI] [PubMed] [Google Scholar]
- Aoki Y., Kanki T., Hirota Y., Kurihara Y., Saigusa T., et al. , 2011. Phosphorylation of serine 114 on Atg32 mediates mitophagy. Mol. Biol. Cell 22: 3206–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J., Wu C., Larocque R., Jamal M., Stevens W., et al. , 2003. Genetic analysis of the interface between Cdc42p and the CRIB domain of Ste20p in Saccharomyces cerevisiae. Genetics 163: 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault A. D., Fassler J. S., Deschenes R. J., 2002. Altered phosphotransfer in an activated mutant of the Saccharomyces cerevisiae two-component osmosensor Sln1p. Eukaryot. Cell 1: 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell L., 2006. Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 34: 837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beese S. E., Negishi T., Levin D. E., 2009. Identification of positive regulators of the yeast Fps1 glycerol channel. PLoS Genet. 5: e1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M., Engelberg D., 2003. Phosphorylation of Tyr-174 of the yeast MAPK Hog1/p38 is not vital for Hog1 biological activity. J. Biol. Chem. 278: 14603–14606 [DOI] [PubMed] [Google Scholar]
- Bell M., Capone R., Pashtan I., Levitzki A., Engelberg D., 2001. Isolation of hyperactive mutants of the MAPK p38/Hog1 that are independent of MAPK kinase activation. J. Biol. Chem. 276: 25351–25358 [DOI] [PubMed] [Google Scholar]
- Bellí G., Garí E., Aldea M., Herrero E., 2001. Osmotic stress causes a G1 cell cycle delay and downregulation of Cln3/Cdc28 activity in Saccharomyces cerevisiae. Mol. Microbiol. 39: 1022–1035 [DOI] [PubMed] [Google Scholar]
- Bender A., Pringle J. R., 1989. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. USA 86: 9976–9980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B. E., Tong J. K., Schreiber S. L., 2000. Genomewide studies of histone deacetylase function in yeast. Proc. Natl. Acad. Sci. USA 97: 13708–13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. B., Gasch A. P., 2008. Stress-activated genomic expression changes serve a preparative role for impending stress in yeast. Mol. Biol. Cell 19: 4580–4587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger B. T., Clark M. G., Amberg D. C., 2007. Requirement for the polarisome and formin function in Ssk2p-mediated actin recovery from osmotic stress in Saccharomyces cerevisiae. Genetics 175: 1637–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjya S., Xu P., Gingras R., Shaykhutdinov R., Wu C., et al. , 2004. Solution structure of the dimeric SAM domain of MAPKKK Ste11 and its interaction with the adaptor protein Ste50 from the budding yeast: implication for Ste11 activation and signal transmission through the Ste50-Ste11 complex. J. Mol. Biol. 344: 1071–1087 [DOI] [PubMed] [Google Scholar]
- Bicknell A. A., Tourtellotte J., Niwa M., 2010. Late phase of the endoplasmic reticulum stress response pathway is regulated by Hog1 MAP kinase. J. Biol. Chem. 285: 17545–17555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland-Marchesan E., Ariño J., Saito H., Sunnerhagen P., Posas F., 2000. Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 20: 3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D., Takahata S., Stillman D. J., 2008. Different genetic functions for the Rpd3(L) and Rpd3(S) complexes suggest competition between NuA4 and Rpd3(S). Mol. Cell. Biol. 28: 4445–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg A., Adler L., 1989. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J. Bacteriol. 171: 1087–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller B., Wanka S., Kraft C., Urban J., Campbell D., et al. , 2010. Phosphoproteomic analysis reveals interconnected system-wide responses to perturbations of kinases and phosphatases in yeast. Sci. Signal. 3: rs4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski G., 1992. PBS2, a yeast gene encoding a putative protein kinase, interacts with the RAS2 pathway and affects osmotic sensitivity of Saccharomyces cerevisiae. J. Gen. Microbiol. 138: 2425–2432 [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72: 743–781 [DOI] [PubMed] [Google Scholar]
- Bouwman J., Kiewiet J., Lindenbergh A., van Eunen K., Iderius M. S., et al. , 2011. Metabolic regulation rather than de novo enzyme synthesis dominates the osmo-adaptation of yeast. Yeast 28: 43–53 [DOI] [PubMed] [Google Scholar]
- Brewster J. L., Gustin M. C., 1994. Positioning of cell growth and division after osmotic stress requires a MAP kinase pathway. Yeast 10: 425–439 [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C., 1993. An osmosensing signal transduction pathway in yeast. Science 259: 1760–1763 [DOI] [PubMed] [Google Scholar]
- Brown J. L., Bussey H., Stewart R. C., 1994. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. EMBO J. 13: 5186–5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns B. R., 2009. The logic of chromatin architecture and remodelling at promoters. Nature 461: 193–198 [DOI] [PubMed] [Google Scholar]
- Capaldi A. P., Kaplan T., Liu Y., Habib N., Regev A., et al. , 2008. Structure and function of a transcriptional network activated by the MAPK Hog1. Nat. Genet. 40: 1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozza M. J., Li B., Florens L., Suganuma T., Swanson S. K., et al. , 2005. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123: 581–592 [DOI] [PubMed] [Google Scholar]
- Casino P., Rubio V., Marina A., 2010. The mechanism of signal transduction by two-component systems. Curr. Opin. Struct. Biol. 20: 763–771 [DOI] [PubMed] [Google Scholar]
- Causton H. C., Ren B., Koh S. S., Harbison C. T., Kanin E., et al. , 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12: 323–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., 2001. HOG on the promoter: regulation of the osmotic stress response. Sci. STKE 93: pe1 [DOI] [PubMed] [Google Scholar]
- Chen R. E., Thorner J., 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1773: 1311–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Gibson T. B., Robinson F., Silvestro L., Pearson G., et al. , 2001. MAP kinases. Chem. Rev. 101: 2449–2476 [DOI] [PubMed] [Google Scholar]
- Chow C. W., Davis R. J., 2006. Proteins kinases: Chromatin-associated enzymes? Cell 127: 887–890 [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Smith K. W., Gustin M. C., 1992. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol. 118: 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotet J., Posas F., 2007. Control of cell cycle in response to osmostress: lessons from yeast. Methods Enzymol. 428: 63–76 [DOI] [PubMed] [Google Scholar]
- Clotet J., Escoté X., Adrover M. A., Yaakov G., Garí E., et al. , 2006. Phosphorylation of Hsl1 by Hog1 leads to a G2 arrest essential for cell survival at high osmolarity. EMBO J. 25: 2338–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Nishikawa J. L., Tang X., Millman J. S., Schub O., et al. , 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117: 899–913 [DOI] [PubMed] [Google Scholar]
- Cross F. R., Schroeder L., Bean J. M., 2007. Phosphorylation of the Sic1 inhibitor of B-type cyclins in Saccharomyces cerevisiae is not essential but contributes to cell cycle robustness. Genetics 176: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Schultz J., Horecka J., Stevenson B. J., Jigami Y., et al. , 2000. Defects in protein glycosylation cause SHO1-dependent activation of a STE12 signaling pathway in yeast. Genetics 155: 1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Sabbagh W., Jr, Graham E., Irick M. M., van Olden E. K., et al. , 2004. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 18: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrcková F., De Virgilio C., Manser E., Pringle J. R., Nasmyth K., 1995. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 9: 1817–1830 [DOI] [PubMed] [Google Scholar]
- Dard N., Peter M., 2006. Scaffold proteins in MAP kinase signaling: more than simple passive activating platforms. Bioessays 28: 146–156 [DOI] [PubMed] [Google Scholar]
- de Bruin R. A. M., McDonald W. H., Kalashnikova T. I., Yates J., III, Wittenberg C., 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117: 887–898 [DOI] [PubMed] [Google Scholar]
- de Nadal E., Posas F., 2008. Regulation of gene expression in response to osmostress by the yeast stress-activated protein kinase Hog1, 81–97 Topics in Current Genetics, Vol. 20, Stress-Activated Protein Kinases, edited by Posas F., Nebreda A. R. Springer-Verlag, Berlin [Google Scholar]
- de Nadal E., Posas F., 2010. Multilayerd control of gene expression by stress-activated protein kinases. EMBO J. 29: 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E., Casadomé L., Posas F., 2003. Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell. Biol. 23: 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E., Zapater M., Alepuz P. M., Sumoy L., Mas G., et al. , 2004. The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427: 370–374 [DOI] [PubMed] [Google Scholar]
- de Nadal E., Ammerer G., Posas F., 2011. Controlling gene expression in response to stress. Nat. Rev. Genet. 12: 833–845 [DOI] [PubMed] [Google Scholar]
- Dihazi H., Kessler R., Eschrich K., 2004. High osmolarity glycerol (HOG) pathway-induced phosphorylation and activation of 6-phosphorfructo-2-kinase are essential for glycerol accumulation and yeast cell proliferation under hyperosmotic stress. J. Biol. Chem. 279: 23961–23968 [DOI] [PubMed] [Google Scholar]
- Dmitrieva N. I., Bulavin D. V., Fornace A. J., Burg M. B., 2002. Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc. Natl. Acad. Sci. USA 99: 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds J. W., Mahadevan L. C., 2006. Cell signaling. Protein kinases seek close encounters with active genes. Science 313: 449–451 [DOI] [PubMed] [Google Scholar]
- Edwards M. C., Liegeoirs N., Horecka J., DePinho R. A., Sprague G. F., Jr, et al. , 1997. Human CPR (cell cycle progression restoration) genes impart a far- phenotype on yeast cells. Genetics 147: 1063–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff S., Murphy S., 2008. Cracking the RNA polymerase II CTD code. Trends Genet. 24: 280–288 [DOI] [PubMed] [Google Scholar]
- Ekiel I., Sulea T., Jansen G., Kowalik M., Minailiuc O., et al. , 2009. Binding the atypical RA domain of Ste50p to the unfolded Opy2p cytoplasmic tail is essential for the high-osmolarity glycerol pathway. Mol. Biol. Cell 20: 5117–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion E. A., 2001. The Ste5p scaffold. J. Cell Sci. 114: 3967–3978 [DOI] [PubMed] [Google Scholar]
- Eraso P., Mazón M. J., Posas F., Portillo F., 2011. Gene expression profiling of yeasts overexpressing wild type or misfolded Pma1 variants reveals activation of the Hog1 MAPK pathway. Mol. Microbiol. 79: 1339–1352 [Google Scholar]
- Escoté X., Zapater M., Clotet J., Posas F., 2004. Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat. Cell Biol. 6: 997–1002 [DOI] [PubMed] [Google Scholar]
- Fassler J. S., West A. H., 2010. Genetic and biochemical analysis of the SLN1 pathway in Saccharomyces cerevisiae. Methods Enzymol. 471: 291–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S., West A. H., 2011. Fungal Skn7 stress responses and their relationship to virulence. Eukaryot. Cell 10: 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C., van Voorst F., Martins A., Neves L., Oliveira R., et al. , 2005. A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol. Biol. Cell 16: 2068–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno P., Posas F., Koepp D., Saito H., Silver P. A., 1998. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J. 17: 5606–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatauer L. J., Zadeh S. F., Bardwell L., 2005. Mitogen-activated protein kinases with distinct requirements for Ste5 scaffolding influence signaling specificity in Saccharomyces cerevisiae. Mol. Cell. Biol. 25: 1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flattery-O’Brien J. A., Dawes I. W., 1998. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J. Biol. Chem. 273: 8564–8571 [DOI] [PubMed] [Google Scholar]
- Fuda N. J., Ardehali M. B., Lis J. T., 2009. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461: 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Hoshi Y., Maeda T., Nakajima T., Abe K., 2005. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 56: 1246–1261 [DOI] [PubMed] [Google Scholar]
- Gao R., Stock A. M., 2009. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63: 133–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gimeno M. A., Struhl K., 2000. Aca1 and Aca2, ATF/CREB activators in Saccharomyces cerevisiae, are important for carbon source utilization but not the response to stress. Mol. Cell. Biol. 20: 4340–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garí E., Volpe T., Wang H., Gallego C., Futcher B., et al. , 2001. Whi3 binds the mRNA of the G1 cyclin CLN3 to modulate cell fate in budding yeast. Genes Dev. 15: 2803–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrenton L. S., Young S. L., Thorner J., 2006. Function of the MAPK scaffold protein, Ste5, requires a cryptic PH domain. Genes Dev. 20: 1946–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A. P., 2007. Comparative genomics of the environmental stress response in ascomycete fungi. Yeast 24: 961–976 [DOI] [PubMed] [Google Scholar]
- Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., et al. , 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11: 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat-Viks I., Shamir R., 2007. Refinement and expansion of signaling pathways: the osmotic response network in yeast. Genome Res. 17: 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M., Tang G., Singleton J., Reményi A., Lim W. A., 2009. The Ste5 scaffold directs mating signaling by catalytically unlocking the Fus3 MAP kinase for activation. Cell 136: 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw S. J., Mott H. R., Stott K. M., Nielsen P. R., Evetts K. A., et al. , 2004. Structure of the sterile α motif (SAM) domain of the Saccharomyces cerevisiae mitogen-activated protein kinase pathway-modulating protein Ste50 and analysis of its interaction with the Ste11 SAM. J. Biol. Chem. 279: 2192–2201 [DOI] [PubMed] [Google Scholar]
- Guha N., Desai P., Vancura A., 2007. Plc1p is required for SAGA recruitment and derepression of Sko1p-regulated genes. Mol. Biol. Cell 18: 2419–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Albertyn J., Alexander M., Davenport K., 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62: 1264–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Lee J.-D., Bibbs L., Ulevitch R. J., 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811 [DOI] [PubMed] [Google Scholar]
- Hao N., Behar M., Parnell S. C., Torres M. P., Borchers C. H., et al. , 2007. A systems-biology analysis of feedback inhibition in the Sho1 osmotic-stress-response pathway. Curr. Biol. 17: 659–667 [DOI] [PubMed] [Google Scholar]
- Hao N., Zeng Y., Elston T. C., Dohlman H. G., 2008. Control of MAPK specificity by feedback phosphorylation of shared adaptor protein Ste50. J. Biol. Chem. 283: 33798–33802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K., Lamson R. E., Nelson B., Hughes T. R., Marton M. J., et al. , 2001. Role of scaffolds in MAP kinase pathway specificity revealed by custom design of pathway-dedicated signaling proteins. Curr. Biol. 11: 1815–1824 [PubMed] [Google Scholar]
- Hayashi M., Maeda T., 2006. Activation of the HOG pathway upon cold stress in Saccharomyces cerevisiae. J. Biochem. 139: 797–803 [DOI] [PubMed] [Google Scholar]
- He X. J., Mulford K. E., Fassler J. S., 2009. Oxidative stress function of the Saccharomyces cerevisiae Skn7 receiver domain. Eukaryot. Cell 8: 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersen P., McClean M. N., Mahadevan L., Ramanathan S., 2008. Signal processing by the HOG MAP kinase pathway. Proc. Natl. Acad. Sci. USA 105: 7165–7170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman M. J., Spatt D., Winston F., 2011. The Hog1 mitogen-activated protein kinase mediates a hypoxic response in Saccharomyces cerevisiae. Genetics 188: 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T., Maeda T., Saito H., Shinozaki K., 1995. Cloning and characterization of seven cDNAs for hyperosmolarity-responsive (HOR) genes of Saccharomyces cerevisiae. Mol. Gen. Genet. 249: 127–138 [DOI] [PubMed] [Google Scholar]
- Hohmann S., 2002a. Osmotic adaptation in yeast: control of the yeast osmolyte system. Int. Rev. Cytol. 215: 149–187 [DOI] [PubMed] [Google Scholar]
- Hohmann S., 2002b. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66: 300–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S., 2009. Control of high osmolarity signalling in the yeast Saccharomyces cerevisiae. FEBS Lett. 583: 4025–4029 [DOI] [PubMed] [Google Scholar]
- Hohmann S., Krantz M., Nordlander B., 2007. Yeast osmoregulation. Methods Enzymol. 428: 29–45 [DOI] [PubMed] [Google Scholar]
- Horie T., Tatebayashi K., Yamada R., Saito H., 2008. Phosphorylated Ssk1 prevents unphosphorylated Ssk1 from activating the Ssk2 MAP kinase kinase kinase in the yeast HOG osmoregulatory pathway. Mol. Cell. Biol. 28: 5172–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler J., Schmid M., Bernard T., Henrissat B., Strahl S., 2007. Membrane association is a determinant for substrate recognition by PMT4 protein O-mannosyltransferases. Proc. Natl. Acad. Sci. USA 104: 7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby T., Flanagan H., Faykin A., Seto A. G., Mattison C., et al. , 1997. Two protein-tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J. Biol. Chem. 272: 17749–17755 [DOI] [PubMed] [Google Scholar]
- Janiak-Spens F., West A. H., 2000. Functional roles of conserved amino acid residues surrounding the phosphorylatable histidine of the yeast phosphorelay protein YPD1. Mol. Microbiol. 37: 136–144 [DOI] [PubMed] [Google Scholar]
- Janiak-Spens F., Sparling J. M., Gurfinkel M., West A. H., 1999. Differential stabilities of phosphorylated response regulator domains reflect functional roles of the yeast osmoregulatory SLN1 and SSK1 proteins. J. Bacteriol. 181: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak-Spens F., Sparling D. P., West A. H., 2000. Novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain. J. Bacteriol. 182: 6673–6678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak-Spens F., Cook P. F., West A. H., 2005. Kinetic analysis of YPD1-dependent phosphotransfer reactions in the yeast osmoregulatory phosphorelay system. Biochemistry 44: 377–386 [DOI] [PubMed] [Google Scholar]
- Jansen G., Bühring F., Hollenberg C. P., Ramezani Rad M., 2001. Mutations in the SAM domain of STE50 differentially influence the MAPK-mediated pathways for mating, filamentous growth and osmotolerance in Saccharomyces cerevisiae. Mol. Genet. Genomics 265: 102–117 [DOI] [PubMed] [Google Scholar]
- Jiang C., Pugh B. F., 2009. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. Genet. 10: 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joaquin M., Gubern A., González-Nuñez D., Josué Ruiz E., Ferreiro I., et al. , 2012. The p57 CDKi integrates stress signals into cell-cycle progression to promote cell survival upon stress. EMBO J. 31: 2952–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P., Tyers M., 2000. The fork’ed path to mitosis. Genome Biol. 1: REVIEWS1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn J. C., ter Riet B., Blad V. S., De Nobel H., Van Den Ende H., et al. , 2001. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 39: 469–479 [DOI] [PubMed] [Google Scholar]
- Kaserer A. O., Andi B., Cook P. F., West A. H., 2009. Effects of osmolytes on the SLN1–YPD1-SSK1 phosphorelay system of Saccharomyces cerevisiae. Biochemistry 48: 8044–8050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaserer A. O., Andi B., Cook P. F., West A. H., 2010. Kinetic studies of the yeast His-Asp phosphorelay signaling pathway. Methods Enzymol. 471: 59–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh M. C., Kurdistani S. K., Morris S. A., Ahn S. H., Podolny V., et al. , 2005. Cotranscriptional Set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123: 593–605 [DOI] [PubMed] [Google Scholar]
- Ketela T., Brown J. L., Stewart R. C., Bussey H., 1998. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Mol. Gen. Genet. 259: 372–378 [DOI] [PubMed] [Google Scholar]
- Kim K. Y., Truman A. W., Levin D. E., 2008. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol. Cell. Biol. 28: 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipp E., Nordlander B., Kruger R., Gennemark P., Hohmann S., 2005. Integrative model of the response of yeast to osmotic shock. Nat. Biotechnol. 23: 975–982 [DOI] [PubMed] [Google Scholar]
- Klopf E., Paskova L., Solé C., Mas G., Petryshyn A., et al. , 2009. Cooperation between the INO80 complex and histone chaperones determines adaptation of stress gene transcription in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 29: 4994–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Inai T., Mizunuma M., Okada I., Shitamukai A., et al. , 2008. Identification of Tup1 and Cyc8 mutations defective in the responses to osmotic stress. Biochem. Biophys. Res. Commun. 368: 50–55 [DOI] [PubMed] [Google Scholar]
- Krantz M., Becit E., Hohmann S., 2006a. Comparative analysis of HOG pathway proteins to generate hypotheses for functional analysis. Curr. Genet. 49: 152–165 [DOI] [PubMed] [Google Scholar]
- Krantz M., Becit E., Hohmann S., 2006b. Comparative genomics of the HOG-signalling system in fungi. Curr. Genet. 49: 137–151 [DOI] [PubMed] [Google Scholar]
- Krantz M., Ahmadpour D., Ottosson L. G., Warringer J., Waltermann C., et al. , 2009. Robustness and fragility in the yeast high osmolarity glycerol (HOG) signal-transduction pathway. Mol. Syst. Biol. 5: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krems B., Charizanis C., Entian K. D., 1996. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr. Genet. 29: 327–334 [DOI] [PubMed] [Google Scholar]
- Kwan J. J., Warner N., Pawson T., Donaldson L. W., 2004. The solution structure of the S. cerevisiae Ste11 MAPKKK SAM domain and its partnership with Ste50. J. Mol. Biol. 342: 681–693 [DOI] [PubMed] [Google Scholar]
- Kwan J. J., Warner N., Maini J., Tung K. W. C., Zakaria H., et al. , 2006. Saccharomyces cerevisiae Ste50 binds the MAPKKK Ste11 through a head-to-tail SAM domain interaction. J. Mol. Biol. 356: 142–154 [DOI] [PubMed] [Google Scholar]
- Lamson R. E., Winters M. J., Pryciak P. M., 2002. Cdc42 regulation of kinase activity and signaling by the yeast p21-activated kinase Ste20. Mol. Cell. Biol. 22: 2939–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson R. E., Takahashi S., Winters M. J., Pryciak P. M., 2006. Dual role for membrane localization in yeast MAP kinase cascade activation and its contribution to signaling fidelity. Curr. Biol. 16: 618–623 [DOI] [PubMed] [Google Scholar]
- Leberer E., Dignard D., Harcus D., Thomas D. Y., Whiteway M., 1992. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein βγ subunits to downstream signalling components. EMBO J. 11: 4815–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Wu C., Leeuw T., Fourest-Lieuvin A., Segall J. E., et al. , 1997. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 16: 83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M., Lu W., Meng W., Parrini M.-C., Eck M. J., et al. , 2000. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102: 387–397 [DOI] [PubMed] [Google Scholar]
- Li B., Carey M., Workman J. L., 2007a. The role of chromatin during transcription. Cell 128: 707–719 [DOI] [PubMed] [Google Scholar]
- Li B., Gogol M., Carey M., Lee D., Seidel C., et al. , 2007b. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science 316: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Li B., Gogol M., Carey M., Pattenden S. G., Seidel C., et al. , 2007c. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev. 21: 1422–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Tsang C. K., Watkins M., Bertram P. G., Zheng X. F., 2006. Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature 442: 1058–1061 [DOI] [PubMed] [Google Scholar]
- Li S., Ault A., Malone C. L., Raitt D., Dean S., et al. , 1998. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17: 6952–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Dean S., Li Z., Horecka J., Deschenes R. J., et al. , 2002. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 13: 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Cai M., 1999. Recovery of the yeast cell cycle from heat shock-induced G1 arrest involves a positive regulation of G1 cyclin expression by the S phase cyclin Clb5. J. Biol. Chem. 274: 24220–24231 [DOI] [PubMed] [Google Scholar]
- Lo W. S., Gamache E. R., Henry K. W., Yang D., Pillus L., et al. , 2005. Histone H3 phosphorylation can promote TBP recruitment through distinct promoter-specific mechanisms. EMBO J. 24: 997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. M. Y., Deschenes R. J., Fassler J. S., 2003. Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot. Cell 2: 1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia J., Regot S., Peeters T., Conde N., Sóle R., et al. , 2009. Dynamic signaling in the Hog1 MAPK pathway relies on high basal signal transduction. Sci. Signal. 2: ra13. [DOI] [PubMed] [Google Scholar]
- MacNeill S. A., Nurse P., 1997. Cell cycle control in fission yeast, pp. 697–763 in The Molecular and Cellular Biology of the Yeast Saccharomyces, Vol. III, Cell Cycle and Cell Biology, edited by J. R. Pringle, J. R. Broach, and E. W. Jones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Maeda T., Tsai A. Y. M., Saito H., 1993. Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol. Cell. Biol. 13: 5408–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Wurgler-Murphy S. M., Saito H., 1994. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369: 242–245 [DOI] [PubMed] [Google Scholar]
- Maeda T., Takekawa M., Saito H., 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269: 554–558 [DOI] [PubMed] [Google Scholar]
- Mager W. H., Varela J. C. S., 1993. Osmostress response of the yeast Saccharomyces. Mol. Microbiol. 10: 253–258 [PubMed] [Google Scholar]
- Maher M., Cong F., Kindelberger D., Nasmyth K., Dalton S., 1995. Cell cycle-regulated transcription of the CLB2 gene is dependent on Mcm1 and a ternary complex factor. Mol. Cell. Biol. 15: 3129–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Wang K., Zhao M., Xu T., Klionsky D. J., 2011. Two MAPK-signaling pathways are required for mitophagy in Saccharomyces cerevisiae. J. Cell Biol. 193: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapes J., Ota I. M., 2004. Nbp2 targets the Ptc1-type 2C Ser/Thr phosphatase to the HOG MAPK pathway. EMBO J. 23: 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marles J. A., Dahesh S., Haynes J., Andrews B. J., Davidson A. R., 2004. Protein-protein interaction affinity plays a crucial role in controlling the Sho1p-mediated signal transduction pathway in yeast. Mol. Cell 14: 813–823 [DOI] [PubMed] [Google Scholar]
- Martín H., Flández M., Nombela C., Molina M., 2005. Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol. Microbiol. 58: 6–16 [DOI] [PubMed] [Google Scholar]
- Martínez-Montañés F., Pascual-Ahuir A., Proft M., 2010. Toward a genomic view of the gene expression program regulated by osmostress in yeast. OMICS 14: 619–627 [DOI] [PubMed] [Google Scholar]
- Martínez-Pastor M. T., Marchler G., Schüller C., Marchler-Bauer A., Ruis H., et al. , 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15: 2227–2235 [PMC free article] [PubMed] [Google Scholar]
- Mas G., de Nadal E., Dechant R., Rodríguez de la Concepción M. L., Logie C., et al. , 2009. Recruitment of a chromatin remodelling complex by the Hog1 MAP kinase to stress genes. EMBO J. 28: 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Muramatsu S., Kamimura Y., Araki H., 2002. S-Cdk-dependent phosphorylation of Sld2 essential for chromosomal DNA replication in budding yeast. Nature 415: 651–655 [DOI] [PubMed] [Google Scholar]
- Mattison C. P., Ota I. M., 2000. Two protein tyrosine phosphatases, Ptp2 and Ptp3, modulate the subcellular localization of the Hog1 MAP kinase in yeast. Genes Dev. 14: 1229–1235 [PMC free article] [PubMed] [Google Scholar]
- McClean M. N., Mody A., Broach J. R., Ramanathan S., 2007. Cross-talk and decision making in MAP kinase pathways. Nat. Genet. 39: 409–414 [DOI] [PubMed] [Google Scholar]
- McMillan J. N., Longtine M. S., Sia R. A., Theesfeld C. L., Bardes E. S., et al. , 1999. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol. 19: 6929–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty J. J., Lew D. J., 2005. Swe1p responds to cytoskeletal perturbation, not bud size, in S. cerevisiae. Curr. Biol. 15: 2190–2198 [DOI] [PubMed] [Google Scholar]
- Mettetal J. T., Muzzey D., Gómez-Uribe C., van Oudenaarden A., 2008. The frequency dependence of osmo-adaptation in Saccharomyces cerevisiae. Science 319: 482–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Schwalb B., Maier K., Schulz D., Dümcke S., et al. , 2011. Dynamic transcriptome analysis measures rates of mRNA synthesis and decay in yeast. Mol. Syst. Biol. 7: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita H., Tsutsui J., Takekawa M., Witten E. A., Saito H., 2002. Regulation of MTK1/MEKK4 kinase activity by its N-terminal domain and GADD45 binding. Mol. Cell. Biol. 22: 4544–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Z., Takekawa M., Ge Q., Saito H., 2007. Activation of MTK1/MEKK4 by GADD45 through induced N-C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol. Cell. Biol. 27: 2765–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody A., Weiner J., Ramanathan S., 2009. Modularity of MAP kinases allows deformation of their signalling pathways. Nat. Cell Biol. 11: 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin C., Jauhiainen A., Warringer J., Nerman O., Sunnerhagen P., 2009. mRNA stability changes precede changes in steady-state mRNA amounts during hyperosmotic stress. RNA 15: 600–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollapour M., Piper P. W., 2006. Hog1p mitogen-activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae. FEMS Yeast Res. 6: 1274–1280 [DOI] [PubMed] [Google Scholar]
- Mollapour M., Piper P. W., 2007. Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol. Cell. Biol. 27: 6446–6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. A., Banks G. R., Toone W. M., Raitt D., Kuge S., et al. , 1997. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16: 1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., 1999. Regulation of the APC and the exit from mitosis. Nat. Cell Biol. 1: E47–E53 [DOI] [PubMed] [Google Scholar]
- Mösch H.-U., Roberts R. L., Fink G. R., 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93: 5352–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Tatebayashi K., Saito H., 2008. Two adjacent docking sites in the yeast Hog1 mitogen-activated protein (MAP) kinase differentially interact with the Pbs2 MAP kinase kinase and the Ptp2 protein tyrosine phosphatase. Mol. Cell. Biol. 28: 2481–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzey D., Gomez-Uribe C. A., Mattetal J. T., van Oudenaarden A., 2009. A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138: 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H., 1992. Yeast SKO1 gene encodes a bZIP protein that binds to the CRE motif and acts as a repressor of transcription. Nucleic Acids Res. 20: 5271–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., Parsons A. B., Evangelista M., Schaefer K., Kennedy K., et al. , 2004. Fus1p interacts with components of the Hog1p mitogen-activated protein kinase and Cdc42p morphogenesis signaling pathways to control cell fusion during yeast mating. Genetics 166: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Bruce C., Hart C., Leigh-Bell J., Gelperin D., et al. , 2009. Dynamic and complex transcription factor binding during an inducible response in yeast. Genes Dev. 23: 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck J., Blomberg A., 1998. Amino acid uptake is strongly affected during exponential growth of Saccharomyces cerevisiae in 0.7 M NaCl medium. FEMS Microbiol. Lett. 158: 121–126 [DOI] [PubMed] [Google Scholar]
- O’Rourke S. M., Herskowitz I., 1998. The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 12: 2874–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke S. M., Herskowitz I., 2002. A third osmosensing branch in Saccharomyces cerevisiae requires the Msb2 protein and functions in parallel with the Sho1 branch. Mol. Cell. Biol. 22: 4739–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke S. M., Herskowitz I., 2004. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell 15: 532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke S. M., Herskowitz I., O’Shea E. K., 2002. Yeast go the whole HOG for the hyperosmotic response. Trends Genet. 18: 405–412 [DOI] [PubMed] [Google Scholar]
- Ostrander D. B., Gorman J. A., 1999. The extracellular domain of the Saccharomyces cerevisiae Sln1p membrane osmolarity sensor is necessary for kinase activity. J. Bacteriol. 181: 2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota I. M., Varshavsky A., 1993. A yeast protein similar to bacterial two-component regulators. Science 262: 566–569 [DOI] [PubMed] [Google Scholar]
- Panadero J., Pallotti C., Rodríguez-Vargas S., Randez-Gil F., Prieto J. A., 2006. A downshift in temperature activates the high osmolarity glycerol (HOG) pathway, which determines freeze tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 281: 4638–4645 [DOI] [PubMed] [Google Scholar]
- Park S.-H., Zarrinpar A., Lim W. A., 2003. Rewiring MAP kinase pathways using alternative scaffold assembly mechanisms. Science 299: 1061–1064 [DOI] [PubMed] [Google Scholar]
- Parmar J. H., Bhartiya S., Venkatesh K. V., 2011. Characterization of the adaptive response and growth upon hyperosmotic shock in Saccharomyces cerevisiae. Mol. BioSyst. 7: 1138–1148 [DOI] [PubMed] [Google Scholar]
- Pascual-Ahuir A., Posas F., Serrano R., Proft M., 2001. Multiple levels of control regulate the yeast cAMP-response element-binding protein repressor Sko1p in response to stress. J. Biol. Chem. 276: 37373–37378 [DOI] [PubMed] [Google Scholar]
- Pascual-Ahuir A., Struhl K., Proft M., 2006. Genome-wide location analysis of the stress-activated MAP kinase Hog1 in yeast. Methods 40: 272–278 [DOI] [PubMed] [Google Scholar]
- Patterson J. C., Klimenko E. S., Thorner J., 2010. Single-cell analysis reveals that insulation maintains signaling specificity between two yeast MAPK pathways with common components. Sci. Signal. 3: ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelet S., Rudolf F., Nadal-Ribelles M., de Nadal E., Posas F., et al. , 2011. Transient activation of the HOG MAPK pathway regulates bimodal gene expression. Science 332: 732–735 [DOI] [PubMed] [Google Scholar]
- Pereira G., Höfken T., Grindlay J., Manson C., Schiebel E., 2000. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell 6: 1–10 [PubMed] [Google Scholar]
- Peter M., Neiman A. M., Park H.-O., van Lohuizen M., Herskowitz I., 1996. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 15: 7046–7059 [PMC free article] [PubMed] [Google Scholar]
- Pitoniak A., Birkaya B., Dionne H. M., Vadaie N., Cullen P. J., 2009. The signaling mucins Msb2 and Hkr1 differentially regulate the filamentation mitogen-activated protein kinase pathway and contribute to a multimodal response. Mol. Biol. Cell 20: 3101–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokholok D. K., Zeitlinger J., Hannett N. M., Reynolds D. B., Young R. A., 2006. Activated signal transduction kinases frequently occupy target genes. Science 313: 533–536 [DOI] [PubMed] [Google Scholar]
- Porter S. W., West A. N., 2005. A common docking site for response regulators on the yeast phosphorelay protein YPD1. Biochim. Biophys. Acta 1748: 138–145 [DOI] [PubMed] [Google Scholar]
- Porter S. W., Xu Q., West A. N., 2003. Ssk1p response regulator binding surface on histidine-containing phosphotransfer protein Ypd1p. Eukaryot. Cell 2: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Saito H., 1997. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: Scaffold role of Pbs2p MAPKK. Science 276: 1702–1705 [DOI] [PubMed] [Google Scholar]
- Posas F., Saito H., 1998. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17: 1385–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Wurgler-Murphy S. M., Maeda T., Witten E. A., Thai T. C., et al. , 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1–YPD1-SSK1 “two-component” osmosensor. Cell 86: 865–875 [DOI] [PubMed] [Google Scholar]
- Posas F., Witten E. A., Saito H., 1998. Requirement of STE50 for osmostress-induced activation of the STE11 mitogen-activated protein kinase kinase kinase in the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 18: 5788–5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Chambers J. R., Heyman J. A., Hoeffler J. P., de Nadal E., et al. , 2000. The transcriptional response of yeast to saline stress. J. Biol. Chem. 275: 17249–17255 [DOI] [PubMed] [Google Scholar]
- Proft M., Struhl K., 2002. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell 9: 1307–1317 [DOI] [PubMed] [Google Scholar]
- Proft M., Struhl K., 2004. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell 118: 351–361 [DOI] [PubMed] [Google Scholar]
- Proft M., Pascual-Ahuir A., de Nadal E., Ariño J., Serrano R., et al. , 2001. Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J. 20: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft M., Gibbons F. D., Copeland M., Roth F. P., Struhl K., 2005. Genomewide identification of Sko1 target promoters reveals a regulatory network that operates in response to osmotic stress in Saccharomyces cerevisiae. Eukaryot. Cell 4: 1343–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft M., Mas G., de Nadal E., Vendrell A., Noriega N., et al. , 2006. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol. Cell 23: 241–250 [DOI] [PubMed] [Google Scholar]
- Pryciak P. M., Huntress F. A., 1998. Membrane recruitment of the kinase cascade scaffold protein Ste5 by the Gβγ complex underlies activation of the yeast pheromone response pathway. Genes Dev. 12: 2684–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao F., Bowie J. U., 2005. The many faces of SAM. Sci. STKE 2005: re7. Review [DOI] [PubMed] [Google Scholar]
- Raitt D. C., Johnson A. L., Erkine A. M., Makino K., Morgan B., et al. , 2000a. The Skn7 response regulator of Saccharomyces cerevisiae intracts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell 11: 2335–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitt D. C., Posas F., Saito H., 2000b. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 19: 4623–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani-Rad M., 2003. The role of adaptor protein Ste50-dependent regulation of the MAPKKK Ste11 in multiple signalling pathways of yeast. Curr. Genet. 43: 161–170 [DOI] [PubMed] [Google Scholar]
- Ramezani Rad M., Xu G., Hollenberg C. P., 1992. STE50, a novel gene required for activation of conjugation at an early step in mating in Saccharomyces cerevisiae. Mol. Gen. Genet. 236: 145–154 [DOI] [PubMed] [Google Scholar]
- Ramezani Rad M., Jansen G., Bühring F., Hollenberg C. P., 1998. Ste50p is involved in regulating filamentous growth in the yeast Saccharomyces cerevisiae and associates with Ste11p. Mol. Gen. Genet. 259: 29–38 [DOI] [PubMed] [Google Scholar]
- Reiser V., Ruis H., Ammerer G., 1999. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell 10: 1147–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Salah S. M., Ammerer G., 2000. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol. 2: 620–627 [DOI] [PubMed] [Google Scholar]
- Reiser V., Raitt D. C., Saito H., 2003. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J. Cell Biol. 161: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., D’Aquino K. E., Ee L. S., Amon A., 2006. The stress-activated mitogen-activated protein kinase signaling cascade promotes exit from mitosis. Mol. Biol. Cell 17: 3136–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reményi A., Good M. C., Bhattacharyya R. P., Lim W. A., 2005. The role of docking interactions in mediating signaling input, output, and discrimination in the yeast MAPK network. Mol. Cell 20: 951–962 [DOI] [PubMed] [Google Scholar]
- Rensing L., Ruoff P., 2009. How can yeast cells decide between three activated MAP kinase pathways? A model approach. J. Theor. Biol. 257: 578–587 [DOI] [PubMed] [Google Scholar]
- Rep M., Albertyn J., Thevelein J. M., Prior B. A., Hohmann S., 1999a. Different signalling pathways contribute to the control of GPD1 gene expression by osmotic stress in Saccharomyces cerevisiae. Microbiology 145: 715–727 [DOI] [PubMed] [Google Scholar]
- Rep M., Reiser V., Gartner U., Thevelein J. M., Hohmann S., et al. , 1999b. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 19: 5474–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep M., Krantz M., Thevelein J. M., Hohmann S., 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock: Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275: 8290–8300 [DOI] [PubMed] [Google Scholar]
- Rep M., Proft M., Remize F., Tamás M., Serrano R., et al. , 2001. The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol. Microbiol. 40: 1067–1083 [DOI] [PubMed] [Google Scholar]
- Robyr D., Suka Y., Xenarios I., Kurdistani S. K., Wang A., et al. , 2002. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109: 437–446 [DOI] [PubMed] [Google Scholar]
- Romero-Santacreu L., Moreno J., Pérez-Ortín J. E., Alepuz P., 2009. Specific and global regulation of mRNA stability during osmotic stress in Saccharomyces cerevisiae. RNA 15: 1110–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., 2001. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem. Rev. 101: 2497–2509 [DOI] [PubMed] [Google Scholar]
- Saito H., 2010. Regulation of cross-talk in yeast MAPK signaling pathways. Curr. Opin. Microbiol. 13: 677–683 [DOI] [PubMed] [Google Scholar]
- Saito H., Tatebayashi K., 2004. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136: 267–272 [DOI] [PubMed] [Google Scholar]
- Sato N., Kawahara H., Toh-e A., Maeda T., 2003. Phosphorelay-regulated degradation of the yeast Ssk1p response regulator by the uniquitin-proteasome system. Mol. Cell. Biol. 23: 6662–6671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A., Core L. J., Lis J. T., 2006. Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell Biol. 7: 557–567 [DOI] [PubMed] [Google Scholar]
- Schaber J., Adrover M. A., Eriksson E., Pelet S., Petelenz-Kurdziel E., et al. , 2010. Biophysical properties of Saccharomyces cerevisiae and their relationship with HOG pathway activation. Eur. Biophys. J. 39: 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaber J., Flöttmann M., Li J., Tiger C. F., Hohmann S., et al. , 2011. Automated ensemble modeling with modelMaGe: analyzing feedback mechanisms in the Sho1 branch of the HOG pathway. PLoS ONE 6: e14791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller G. E., Shiu S. H., Armitage J. P., 2011. Two-component systems and their co-option for eukaryotic signal transduction. Curr. Biol. 21: R320–R330 [DOI] [PubMed] [Google Scholar]
- Schmitt A. P., McEntee K., 1996. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93: 5777–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Madhani H. D., 2004. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu. Rev. Genet. 38: 725–748 [DOI] [PubMed] [Google Scholar]
- Schwob E., Böhm T., Mendenhall M. D., Nasmyth K., 1994. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell 79: 233–244 [DOI] [PubMed] [Google Scholar]
- Shahbazian M. D., Grunstein M., 2007. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 76: 75–100 [DOI] [PubMed] [Google Scholar]
- Shankarnarayan S., Malone C. L., Deschenes R. J., Fassler J. S., 2008. Modulation of yeast Sln1 kinase activity by the Ccw12 cell wall protein. J. Biol. Chem. 283: 1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Hamad D., Gustin M. C., 2004. MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am. J. Physiol. Renal Physiol. 287: F1102–F1110 [DOI] [PubMed] [Google Scholar]
- Shivaswamy S., Iyer V. R., 2008. Stress-dependent dynamics of global chromatin remodeling in yeast: dual role for SWI/SNF in the heat shock stress response. Mol. Cell. Biol. 28: 2221–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock T. R., Thompson J., Yates J. R., Madhani H. D., 2009. Hog1 mitogen-activated protein kinase (MAPK) interrupts signal transduction between the Kss1 MAPK and the Tec1 transcription factor to maintain pathway specificity. Eukaryot. Cell 8: 606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderius M., Van Wuytswinkel O., Reijenga K. A., Kelders M., Mager W. H., 2000. The control of intracellular glycerol in Saccharomyces cerevisiae influences osmotic stress response and resistance to incrased temperature. Mol. Microbiol. 36: 1381–1390 [DOI] [PubMed] [Google Scholar]
- Solé C., Nadal-Ribelles M., Kraft C., Peter M., Posas F., et al. , 2011. Control of Ubp3 ubiquitin protease activity by the Hog1 SAPK modulates transcription upon osmostress. EMBO J. 30: 3274–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. K., Lee J. Y., Lee M. G., Moon J., Min K., et al. , 1999. Insights into eukaryotic multistep phosphorelay signal transduction revealed by the crystal structure of Ypd1 from Saccharomyces cerevisiae. J. Mol. Biol. 293: 753–761 [DOI] [PubMed] [Google Scholar]
- Sotelo J., Rodríguez-Gabriel M. A., 2006. Mitogen-activated protein kinase Hog1 is essential for the response to arsenite in Saccharomyces cerevisiae. Eukaryot. Cell 5: 1826–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi B., Kelstrup C. D., Stoehr G., Fröhlich F., Walther T. C., et al. , 2009. Global analysis of the yeast osmotic stress response by quantitative proteomics. Mol. Biosyst. 5: 1337–1346 [DOI] [PubMed] [Google Scholar]
- Sprague G. F., 1998. Control of MAP kinase signaling specificity or how not to go HOG wild. Genes Dev. 12: 2817–2820 [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Amon A., 2004. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38: 203–232 [DOI] [PubMed] [Google Scholar]
- Stock A. M., Robinson V. L., Goudreau P. N., 2000. Two-component signal transduction. Annu. Rev. Biochem. 69: 183–215 [DOI] [PubMed] [Google Scholar]
- Takekawa M., Saito H., 1998. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95: 521–530 [DOI] [PubMed] [Google Scholar]
- Takekawa M., Posas F., Saito H., 1997. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 16: 4973–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamás M. J., Luyten K., Sutherland F. C., Hernandez A., Albertyn J., et al. , 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31: 1087–1104 [DOI] [PubMed] [Google Scholar]
- Tamás M. J., Rep M., Thevelein J. M., Hohmann S., 2000. Stimulation of the yeast high osmolarity glycerol (HOG) pathway: evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett. 472: 159–165 [DOI] [PubMed] [Google Scholar]
- Tanaka S., Umemori T., Hirai K., Muramatsu S., Kamimura Y., et al. , 2007. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 445: 328–332 [DOI] [PubMed] [Google Scholar]
- Tanigawa M., Kihara A., Terashima M., Takahara T., Maeda T., 2012. Sphingolipids regulate the yeast high osmolarity-responsive HOG pathway. Mol. Cell. Biol. 32: 2861–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W., Deschenes R. J., Fassler J. S., 1999. Intracellular glycerol levels modulate the activity of Sln1p, a Saccharomyces cerevisiae two-component regulator. J. Biol. Chem. 274: 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K., Takekawa M., Saito H., 2003. A docking site determining specificity of Pbs2 MAPKK for Ssk2/Ssk22 MAPKKKs in the yeast HOG pathway. EMBO J. 22: 3624–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K., Yamamoto K., Tanaka K., Tomida T., Maruoka T., et al. , 2006. Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway. EMBO J. 25: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K., Tanaka K., Yang H.-Y., Yamamoto K., Matsushita Y., et al. , 2007. Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J. 26: 3521–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M., Scheikl E., Reiser V., Ruis H., Ammerer G., 2001. Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc. Natl. Acad. Sci. USA 98: 5625–5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen M., Di Y., Tängemo C., Morillas M., Ahmadpour D., et al. , 2006. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol. Biol. Cell 17: 4400–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandra H., Compton J., Kellogg D., 1998. Control of mitotic events by the Cdc42 GTPase, the Clb2 cyclin and a member of the PAK kinase family. Curr. Biol. 8: 991–1000 [DOI] [PubMed] [Google Scholar]
- Toh-e A., Oguchi T., 2001. Defects in glycosylphosphatidylinositol (GPI) anchor synthesis activate Hog1 kinase and confer copper-resistance in Saccharomyces cerevisiae. Genes Genet. Syst. 76: 393–410 [DOI] [PubMed] [Google Scholar]
- Tokishita S., Mizuno T., 1994. Transmembrane signal transduction by the Escherichia coli osmotic sensor, EnvZ: intermolecular complementation of transmembrane signalling. Mol. Microbiol. 13: 435–444 [DOI] [PubMed] [Google Scholar]
- Tomás-Cobos L., Casadomé L., Mas G., Sanz P., Posas F., 2004. Expression of the HXT1 low affinity glucose transporter requires the coordinated activities of the HOG and glucose signalling pathways. J. Biol. Chem. 279: 22010–22019 [DOI] [PubMed] [Google Scholar]
- Torres-Quiroz F., García-Marqués S., Coria R., Randez-Gil F., Prieto J. A., 2010. The activity of yeast Hog1 MAPK is required during endoplasmic reticulum stress induced by tunicamycin exposure. J. Biol. Chem. 285: 20088–20096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truckses D. M., Bloomekatz J. E., Thorner J., 2006. The RA domain of Ste50 adaptor protein is required for delivery of Ste11 to the plasma membrane in the filamentous growth signaling pathway of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 912–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesono Y., Toh-e A., 2002. Transient inhibition of translation initiation by osmotic stress. J. Biol. Chem. 277: 13848–13855 [DOI] [PubMed] [Google Scholar]
- Vadaie N., Dionne H., Akajagbor D. S., Nickerson S. R., Krysan D. J., et al. , 2008. Cleavage of the signaling mucin Msb2 by the aspartyl protease Yps1 is required for MAPK activation in yeast. J. Cell Biol. 181: 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drogen F., O’Rourke S. M., Stucke V. M., Jaquenoud M., Neiman A. M., et al. , 2000. Phosphorylation of the MEKK Ste11p by the PAK-like kinase Ste20p is required for MAP kinase signaling in vivo. Curr. Biol. 10: 630–639 [DOI] [PubMed] [Google Scholar]
- Vasudevan S., Peltz S. W., 2001. Regulated ARE-mediated mRNA decay in Saccharomyces cerevisiae. Mol. Cell 7: 1191–1200 [DOI] [PubMed] [Google Scholar]
- Vendrell A., Martínez-Pastor M., González-Novo A., Pascual-Ahuir A., Sinclair D. A., et al. , 2011. Sir2 histone deacetylase prevents programmed cell death caused by sustained activation of the Hog1 stress-activated protein kinase. EMBO Rep. 12: 1062–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Annan R. S., Huddleston M. J., Carr S. A., Reynard G., et al. , 1997. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science 278: 455–460 [DOI] [PubMed] [Google Scholar]
- Vincent A. C., Struhl K., 1992. ACR1, a yeast ATF/CREB repressor. Mol. Cell. Biol. 12: 5394–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. C., Morgan L. K., Godakumbura P., Kenney L. J., Anand G. S., 2012. The inner membrane histidine kinase EnvZ senses osmolality via helix-coil transitions in the cytoplasm. EMBO J. 31: 2648–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., McGowan C. H., Zhao M., He L., Downey J. S., et al. , 2000. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20: 4543–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Irqeba A. A., Ayalew M., Suntay K., 2009. Sumoylation of transcription factor Tec1 regulates signaling of mitogen-activated protein kinase pathways in yeast. PLoS ONE 4: e7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmka J., Hanneman J., Lee J., Amin D., Ota I., 2001. Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 21: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warringer J., Hult M., Regot S., Posas F., Sunnerhagen P., 2010. The HOG pathway dictates the short-term translational response after hyperosmotic shock. Mol. Biol. Cell 21: 3080–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Srichuwong S., Arakane M., Tamiya S., Yoshinaga M., et al. , 2010. Selection of stress-tolerant yeasts for simultaneous saccharification and fermentation (SSF) of very high gravity (VHG) potato mash to ethanol. Bioresour. Technol. 101: 9710–9714 [DOI] [PubMed] [Google Scholar]
- Wertman K. F., Drubin D. G., Botstein D., 1992. Systematic mutational analysis of the yeast ACT1 gene. Genetics 132: 337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall P. J., Thorner J., 2006. Analysis of mitogen-activated protein kinase signaling specificity in response to hyperosmotic stress: use of an analog-sensitive HOG1 allele. Eukaryot. Cell 5: 1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall P. J., Patterson J. C., Chen R. E., Thorner J., 2008. Stress resistance and signal fidelity independent of nuclear MAPK function. Proc. Natl. Acad. Sci. USA 105: 12212–12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A., Arkind C., Mattison C. P., Burkholder A., Knoche K., et al. , 2002. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell 1: 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters M. J., Lamson R. E., Nakanishi H., Neiman A. M., Pryciak P. M., 2005. A membrane binding domain in the Ste5 scaffold synergizes with Gβγ binding to control localization and signaling in pheromone response. Mol. Cell 20: 21–32 [DOI] [PubMed] [Google Scholar]
- Wood J. M., 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63: 230–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., 2011. Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu. Rev. Microbiol. 65: 215–238 [DOI] [PubMed] [Google Scholar]
- Wu C., Leberer E., Thomas D. Y., Whiteway M., 1999. Functional characterization of the interaction of Ste50p with Ste11p MAPKKK in Saccharomyces cerevisiae. Mol. Biol. Cell 10: 2425–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Jansen G., Zhang J., Thomas D. Y., Whiteway M., 2006. Adaptor protein Ste50p links the Ste11p MEKK to the HOG pathway through plasma membrane association. Genes Dev. 20: 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurgler-Murphy S. M., Maeda T., Witten E. A., Saito H., 1997. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol. 17: 1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Jansen G., Thomas D. Y., Hollenberg C. P., Ramezani Rad M., 1996. Ste50p sustains mating pheromone-induced signal transduction in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 20: 773–783 [DOI] [PubMed] [Google Scholar]
- Xu Q., West A. H., 1999. Conservation of structure and function among histidine-containing phosphotransfer (HPt) domains as revealed by the crystal structure of YPD1. J. Mol. Biol. 292: 1039–1050 [DOI] [PubMed] [Google Scholar]
- Xu Q., Porter S. W., West A. N., 2003. The yeast YPD1/SLN1 complex: insights into molecular recognition in two-component signaling systems. Structure 11: 1569–1581 [DOI] [PubMed] [Google Scholar]
- Yaakov G., Bell M., Hohmann S., Engelberg D., 2003. Combination of two activating mutations in one HOG1 gene forms hyperactive enzyme that induce growth arrest. Mol. Cell. Biol. 23: 4826–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakov G., Duch A., García-Rubio M., Clotet J., Jimenez J., et al. , 2009. The stress-activated protein kinase Hog1 mediates S phase delay in response to osmostress. Mol. Biol. Cell 20: 3572–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Tatebayashi K., Tanaka K., Saito H., 2010. Dynamic control of yeast MAP kinase network by induced association and dissociation between the Ste50 scaffold and the Opy2 membrane anchor. Mol. Cell 40: 87–98 [DOI] [PubMed] [Google Scholar]
- Yancey P. H., 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208: 2819–2830 [DOI] [PubMed] [Google Scholar]
- Yang H.-Y., Tatebayashi K., Yamamoto K., Saito H., 2009. Glycosylation defects activate filamentous growth Kss1 MAPK and inhibit osmoregulatory Hog1 MAPK. EMBO J. 28: 1380–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. J., Seto E., 2008. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9: 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Phadtare S., Inouye M., 2007. Functional and structural characterization of EnvZ, an osmosensing histidine kinase of E. coli. Methods Enzymol. 423: 184–202 [DOI] [PubMed] [Google Scholar]
- Young C., Mapes J., Hanneman J., Al-Zarban S., Ota I., 2002. Role of Ptc2 type 2C Ser/Thr phosphatase in yeast high-osmolarity glycerol pathway inactivation. Eukaryot. Cell 1: 1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk T., Amberg D. C., 2003. Actin recovery and bud emergence in osmotically stressed cells requires the conserved actin interacting mitogen-activated protein kinase kinase kinase Ssk2p/MTK1 and the scaffold protein Spa2p. Mol. Biol. Cell 14: 3013–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk T., Foehr M., Amberg D. C., 2002. The MEK kinase Ssk2p promotes actin cytoskeleton recovery after osmotic stress. Mol. Biol. Cell 13: 2869–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S., Lippman S. I., Zhao X., Broach J. R., 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42: 27–81 [DOI] [PubMed] [Google Scholar]
- Zapater M., Clotet J., Escoté X., Posas F., 2005. Control of cell cycle progression by the stress-activated Hog1 MAPK. Cell Cycle 4: 6–7 [DOI] [PubMed] [Google Scholar]
- Zapater M., Sohrmann M., Peter M., Posas F., de Nadal E., 2007. Selective requirement for SAGA in Hog1-mediated gene expression depending on the severity of the external osmostress conditions. Mol. Cell. Biol. 27: 3900–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A., Park S.-H., Lim W. A., 2003. Optimization of specificity in a cellular protein interaction network by negative selection. Nature 426: 676–680 [DOI] [PubMed] [Google Scholar]
- Zarrinpar A., Bhattacharyya R. P., Nittler M. P., Lim W. A., 2004. Sho1 and Pbs2 act as coscaffolds linking components in the yeast high osmolarity MAP kinase pathway. Mol. Cell 14: 825–832 [DOI] [PubMed] [Google Scholar]
- Zhan X.-L., Guan K.-L., 1999. A specific protein-protein interaction accounts for the in vivo substrate selectivity of Ptp3 towards the Fus3 MAP kinase. Genes Dev. 13: 2811–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Copeland D. M., Soares A. S., West A. H., 2008. Crystal structure of a complex between the phosphorelay protein YPD1 and the response regulator domain of SLN1 bound to a phosphoryl analog. J. Mol. Biol. 375: 1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zi Z., Liebermeister W., Klipp E., 2010. A quantitative study of the Hog1 MAPK response to fluctuating osmotic stress in Saccharomyces cerevisiae. PLoS ONE 5: e9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzalla V., Graziola M., Mastriani A., Vanoni M., Alberghina L., 2007. Rapamycin-mediated G1 arrest involves regulation of the Cdk inhibitor Sic1 in Saccharomyces cerevisiae. Mol. Microbiol. 63: 1482–1494 [DOI] [PubMed] [Google Scholar]
- Zou X., Peng T., Pan Z., 2007. Modeling specificity in the yeast MAPK signaling networks. J. Theor. Biol. 250: 139–155 [DOI] [PubMed] [Google Scholar]