Abstract
Purpose
Failed implantation is a major limiting factor in infertility and early pregnancy loss. In primates, human chorionic gonadotropin mediated inhibition of stromal cell apoptosis and their subsequent differentiation into decidual cells is critical for successful embryo implantation. A major regulator of cell survival and differentiation is the Notch receptor, which transduces extracellular signals responsible for cell fate determination during development. Proteolytic cleavage of full-length Notch1 releases an active intracellular peptide, which later translocates to the nucleus and activates gene transcription. Induction of Notch1 during the window of uterine receptivity in stromal fibroblasts in response to chorionic gonadotropin upregulates anti- apoptotic genes and induces α-smooth muscle actin, enabling stromal cells to proliferate and differentiate into a decidualized phenotype. As such, prior to implantation the embryonic signal, chorionic gonadotropin, rescues stromal fibroblasts from normal regression at the end of each ovarian cycle.
Conclusion
We are suggesting that chorionic gonadotropin and Notch1 coordinately regulate decidualization by preventing apoptosis of endometrial stromal fibroblasts, averting uterine sloughing, and promoting cell survival and differentiation into the decidualized phenotype, which is critical for the maintenance of pregnancy.
Keywords: Chorionic gonadotropin, Decidualization, Implantation, Notch1, Uterine receptivity
Introduction
Failed implantation is a major limiting factor in assisted reproduction; these events are not clinically recognized as pregnancies [1]. As so, of the pregnancies that are lost, 50–75% represent a failure of implantation [2, 3]. Determining the prevalence of miscarriages is difficult, as many occur even before a woman is aware that she is pregnant [4]. However, prospective studies have found that 25% of pregnancies are miscarried by the sixth week of the last menstrual period [5] and only one-half of all conceptions advance beyond 20 weeks of gestation [6, 7]. Furthermore, by the age of 45, 75% of pregnancies end in miscarriages [8]. A better understanding of the molecular mechanisms responsible for implantation may improve clinician’s ability to treat infertility and early pregnancy loss.
One of the most important requirements for the mammalian embryo to establish a successful pregnancy is its ability to implant in the mother’s uterus. This process implies an appropriate spatio-temporal synchronic series of events where the embryonic trophoblast establishes contact with the uterus, attaching and intruding to the luminal epithelium. In order to carry out this dialogue, the endometrium must become receptive to signals derived from the embryo. This review explores the correlation between chorionic gonadotropin (CG) and Notch1 during the window of uterine receptivity. We suggest that CG and Notch1 coordinately regulate decidualization by preventing apoptosis of endometrial stromal fibroblasts, averting uterine sloughing, and promoting cell survival and differentiation into the decidualized phenotype, which is critical for the maintenance of pregnancy.
Chorionic gonadotropin
In order to maintain the corpus luteum of pregnancy, different species utilize diverse mechanisms. In the primate, CG is one of the early embryonic signals secreted from the trophoblast cells of the pre-implantation embryo. CG has a direct luteotrophic effect on the corpus luteum, increasing its life span. Concurrently, CG also leads to alterations in morphology and endometrial gene expression by modulating the endometrium in preparation for implantation [9].
Our work has shown that infusion of CG into the uterine cavity of cycling baboons, in a manner which mimics normal blastocyst transit, induces the induction of α-smooth muscle actin (α-SMA) in the stromal fibroblasts. Disruption of α-SMA leads to apoptosis and a decrease in Notch1. Work from our laboratory has shown that treatment with CG rescues these cells from apoptosis and induces Notch1 expression pointing towards the critical role of both CG and Notch1 processes in implantation.
Molecular biology of CG
The CG receptor is ubiquitously expressed in reproductive organs [10, 11]. Targeted deletion of the CG receptor results in an altered endometrial phenotype and disrupted gene expression [12]. More so, CG receptor null animals do not demonstrate a complete decidualization response and have lower implantation rates when wild type donor embryos were transferred into the uterus [13]. Luteinizing hormone β (LH-β) knockout mice have been created and have thin uteri. These transgenic females are hypogonadal and infertile. Serum progesterone and estradiol are decreased by 75% and the uteri are hypoplastic consisting of thin endometrial layer [14]. Noteworthy, is the fact that pharmacological rescue of this phenotype was achieved with treatment of CG. Moreover, another LH-receptor knockout (LuRKO), consistent with the LH-β knockouts harbor thin uteri, with limited glands in the endometrium [15].
During implantation and pregnancy CG is primarily secreted by the syncytiotrophoblast and is involved in creating a receptive endometrium by directly modulating the function of both endometrial stromal and epithelial cells [16]. CG is a glycoprotein hormone belonging to the same family as follicle stimulating hormone (FSH), luteinizing hormone (LH) and thyroid stimulating hormone (TSH). The LH/CG receptor is a large cell-surface glycoprotein with the characteristic structure that makes it a member of the superfamily of G protein-coupled receptors (GPCR) [17]. These receptors activate multiple pathways in different cells, acting through various subunits and subsequent second messengers. The LH/CG receptor is composed of 11 exons, and has been mapped to 2p21 [18, 19]. The terminal exon encodes the entire carboxyl-terminal half of the receptor, including all seven transmembrane helices, the three interconnecting extracellular loops, the three interconnecting intracellular loops and the cytoplasmic tail. This carboxyl half of the receptor shares homology with other members of the superfamily of rhodopsin-like G protein-coupled receptors. The first 10 exons of the LH/CG receptor gene encode a large amino-terminal extracellular domain that contains a number of leucine-rich repeat motifs likely to be involved in protein–protein interactions. The extracellular domain of the LH/CG receptor, when expressed in isolation in transfected cells, binds CG with the same high affinity, as does the full-length receptor. Thus, although the extracellular N-terminal domain of the receptor mediates the high-affinity binding of the hormone it does not preclude low affinity interactions with the carboxyl half of the receptor [20].
The LH/CG receptor, like other GPCR undergoes ligand-induced desensitization, becoming less responsive to stimuli within minutes after binding the ligand [21]. Similarly, as pregnancy progresses, subsequent decidualization results in the downregulation of the LH/CG receptor, thought to be a prerequisite for the morphological transformation of stromal cells in the endometrium. This reduction in receptor expression corresponds to a decrease in CG in peripheral circulation [22].
We have investigated the cross talk between CG and progesterone in the endometrium during the period of receptivity. Our results indicate that blocking the action of progesterone on the endometrium, even briefly, has overwhelming effects on the CG-induced response in stromal fibroblasts. In contrast, the diminution of epithelial function in the presence of an ovary requires prolonged inhibition of progesterone action, suggesting a potential paracrine effect on the endometrium from the corpus luteum in response to CG.
Notch1
Uterine and embryonic interactions during implantation are correlative to epithelial–mesenchymal interactions in embryogenesis, and thus involve evolutionary conserved genes across species [23]. The Notch family of receptors mediate a highly conserved pathway that regulates differentiation and pro-survival signals across species from invertebrates to humans [24]. Notch proteins are ligand-dependant transmembrane receptors that transduce extracellular signals responsible for cell-fate and differentiation throughout development [25, 26]. Notch signaling often restricts the differentiation fates of a cell, directing it to a specific cell fate in cooperation with other signals, while at the same time inhibiting differentiation toward an alternate fate and promoting survival [27].
Experimental evidence indicates that Notch signaling regulates all three branches of the cell fate decision tree: differentiation, cell cycle progression and apoptotic cell death [25, 27, 28]. The effects of Notch signaling on cell fate decisions in vertebrates have been extensively studied in tissue culture, ex vivo systems and transgenic animals [29–32].
Molecular biology of Notch1
The Notch proteins encompass a family of four homologous heterodimeric transmembrane receptors. Ligand-activated proteolytic cleavage of full-length Notch releases an active intracellular peptide, which later translocates to the nucleus and activates gene transcription [33]. Synthesized as a single polypeptide precursor in the endoplasmic reticulum, the Notch receptor is cleaved into a bipartite protein by a furin-like convertase in the trans-Golgi [34, 35]. These transmembrane receptors are made up of an extracellular and transmembrane subunits non-covalently held together by calcium-dependant interactions. Ligand binding at the extracellular domain results in dissociation of the extracellular subunit from the transmembrane subunit, followed by two sequential cleavages of Notch. The first is catalyzed by an ADAM protease and the second by γ-secretase [36]. This latter cleavages releases the active intracellular domain (NICD), which migrates to the nucleus and activates transcription by binding to ubiquitous transcription factor CBF1/RBPJκ and recruiting coactivator MAML1 (mastermind-like 1). The extracellular subunit of Notch receptors contains tandem epidermal growth-factor-like repeats that are involved in ligand recognition [37, 38]. The transmembrane domain includes a RAM domain that interacts with CBF1/RBPJκ [39]. This causes dissociation of a large co-repressor complex, which is replaced by a coactivator complex including MAML1, triggering transcriptional activation of Notch target genes. After transcriptional activation the C-terminal PEST sequence in Notch NICD gets ubiqutiinated and triggers receptor turnover [40]. Ligands for Notch are members of the DSL (Delta, Serrate, Lag-2) family of transmembrane proteins. The five mammalian ligands include: Jagged1, Jagged2, Delta1, Delta3, Delta4 [41]. Like the Notch receptors, these ligands have an extracellular domain comprised primarily of EGF-like repeats.
Notch1 knockouts are embryonic lethal because of vascular and somatic defects [42, 43]. It has been shown in mice that Notch1 is essential for post-implantation development. Crossing animals heterozygous for the Notch1 mutation yields mostly non-viable offspring and embryos that did survive beyond 11.5 days of gestation revealed widespread cell death, not attributable to defects in vascularization [44]. Similarly, targeted disruption of Notch ligand genes result in severe developmental defects or embryonic lethality [45, 46].
Window of uterine receptivity
After fertilization, the conceptus begins its migration from the oviduct into the uterus where it attempts to implant. Implantation requires a series of very precisely timed responses from both the embryo and the uterus. This occurs within a short period of time, known as the window of uterine receptivity. If implantation is successful, the endometrial stromal compartment forms the decidua, a morphologically and functionally gestation-specific tissue, representing the maternal side of the feto-maternal interface. This transformation requires a modified uterine milieu that promotes differentiation and metabolically active cells called decidual cells.
The concept of endometrial receptivity was first established in rodents and then extended to other species [47]. Although estrogen and progesterone have long been believed to be essential for developing an appropriate endometrial environment for blastocyst implantation, it is now evident that peptide hormones, such as CG, and growth factors further modulate these effects secreted by a variety of cell types within the uterine endometrium. In preparation for the implanting blastocyst, the endometrium becomes increasingly vascular, inducing α-SMA in stromal fibroblasts. More so, endometrial glands respond to CG by displaying an enhanced secretory activity and a plaque response develops in the luminal epithelium. The window of receptivity ranges from day 6 to day 10 post-ovulation in primates.
In addition to the quality of the embryo, inhibition of stromal cell apoptosis by (h)CG, and subsequent cell differentiation into fully differentiated decidual cells is critical for successful implantation. Differentiation into decidual cells is important for control of endometrial vascularization and involves vasodilation and angiogenesis in the endometrium [48]. During the process of decidualization, endometrial stromal fibroblasts transform morphologically and biochemically into polygonal, secretory cells and begin to express specific decidual proteins such as prolactin and insulin like growth factor binding protein-1 (IGFBP-1) [49]. Although the morphological changes are useful predictors of successful implantation, the molecular mechanisms underlying them are largely unknown and remain to be elucidated.
Notch activation modulates the response of a cell to a differentiation stimulus, rather than directly specifying cell fate. In some cell types, as initially discovered in murine erythroloeukemia cells, downregulation of Notch1 during differentiation abrogates differentiation, and results in subsequent apoptosis [50]. Activation of Notch1 provides a temporary survival signal during the differentiation of many cells, including thymocytes [30], murine erythroleukemia cells [50], murine and human keratinocytes [51].
Notch1 has both dose- and time-dependent effects on the levels of apoptotic inhibitor Bcl-xL and cell cycle regulators p21 (cip1/waf1), p27 (kip1), and Rb [52, 53]. Additionally, Notch1 activation by a cell-associated ligand is accompanied by a rapid and transient induction of NFκB DNA binding activity, which is known to regulate anti-apoptotic genes [54, 55]. The relative effects of Notch1 signaling on these pathways depend on the levels of Notch1 expression, the mechanism of activation, and the timing of activation.
Transforming the uterus to a receptive uterine stromal environment from the pre-menses state involves the synchronized regulation of CG, ovarian hormones and Notch1. Work in our laboratory has shown that (h)CG and ovarian steroids induce Notch1 expression in preventing stromal cell apoptosis and regression that occurs in the non-pregnant woman at the onset of the menstrual cycle. Subsequently, Notch1 expression is downregulated to allow stromal cell differentiation to make way for the decidualization phenotype.
In summary, our data show that low expression of Notch1 can be correlated to shedding of the uterine lining and an inability of the uterus to accept an implanting embryo, making it tempting to speculate that the ability levels of Notch1 can be used as an early infertility marker in women undergoing multiple miscarriages.
Role of the cytoskeleton
A coordinated process of programmed cell death is involved in both embryonic development and tissue homeostasis in the adult and as such is an important regulator of endometrial function. Apoptosis was first described in the human endometrium in 1975 [56] and subsequently shown to be rare in the proliferative endometrium, whereas levels of apoptosis increase in secretory tissue and peaks during the menstrual phase [57, 58]. Treatment with (h)CG or progesterone in the mid-secretory phase inhibits apoptosis in late secretory phase, implying a role for embryonic signals in inhibiting apoptosis [59].
The cytoskeleton plays a decisive role in mitosis, cell growth, cell motility and inhibition of apoptosis [60, 61]. Studies from our laboratory indicate that the primary effect of (h)CG on stromal fibroblasts is the induction of αSMA, which is a consequence of integrins on the stromal cell membrane binding to secreted extracellular matrix proteins [16, 62–64]. We have demonstrated that the induction of αSMA by (h)CG may be essential in order to decrease the progesterone-regulated proliferation in these cells, and consequently initiating differentiation and a qualification to inhibit stromal cell apoptosis [65]. This inhibition of proliferation in response to (h)CG has also been documented in human breast epithelial cells and breast cancer cells in culture [66]. In addition, co-expression of both the (h)CG receptor and COX-2 in stromal cells at the implantation site during early pregnancy is in agreement with previous in vitro studies that demonstrated a direct effect of (h)CG on COX-2 gene expression and the induction of decidualization in human stromal cells cultured in vitro [67].
Noseda et al. [68, 69] have demonstrated the requirement of Notch activation on the regulation of αSMA expression levels, which are coordinately regulated in decidualization. Notch was shown to directly regulate expression of the mesenchymal and smooth muscle cell marker αSMA by activating a cis element consensus sequence on a transcriptional effector that is required for Notch-mediated αSMA induction. Furthermore, evidence in our baboon model indicates that coincident with the induction of αSMA, Notch1 and its ligand Jagged1 are also expressed in stromal fibroblasts.
As a consequence of implantation, apoptosis is avoided, and so are the subsequent sloughing off of endometrial tissue and disruption of spiral artery integrity that are hallmarks of menstruation. Studies in human endometrial fibroblasts with an apoptosis-inducing agent which disrupts the actin microfilaments, cytochalasin D indicate that (h)CG can significantly inhibit cytochalasin D-induced apoptosis of stromal cells, and this effect is enhanced in the presence of estrogen and progesterone [70]. Studies in human endometrial tissue suggest that ovarian steroids regulate three apoptotic related proteins (Bcl-2, Bcl-X and Bax) [59, 71, 72]. Thus, (h)CG and progesterone significantly reduce apoptosis in the endometrium. Furthermore, in vivo treatment of women with (h)CG or progesterone significantly increased Bcl-2 in the late luteal phase. Bcl-X levels were higher in the (h)CG treated group compared to the progesterone treated group, while Bax was inhibited by progesterone primarily [59]. Notch1 has also been shown to up regulate the anti-apoptotic protein, Bcl-XL [52], suggesting that it may participate in this effect of (h)CG.
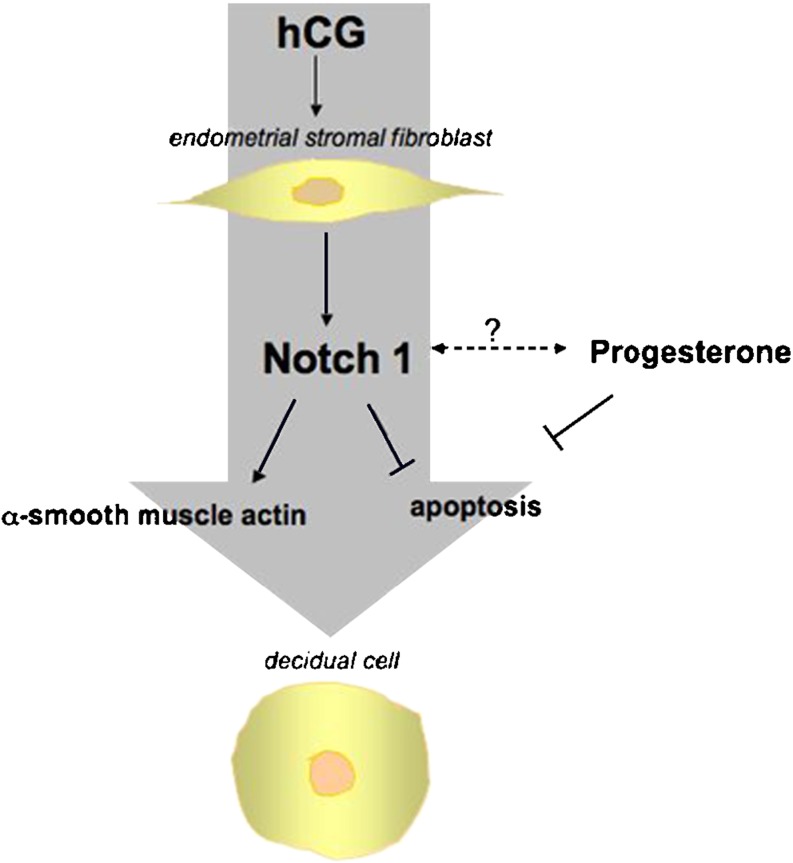
These observations suggest that (h)CG induces αSMA and Notch1, which act synergistically on stromal fibroblasts during the window of uterine receptivity to inhibit apoptosis and enhance differentiation by upregulating anti-apoptotic genes [73, 74] (Fig. 1).
Fig. 1.
The induction of Notch1 in stromal fibroblasts in response to hCG and progesterone inhibits apoptosis induces α-smooth muscle actin and enables the stromal cells to proliferate and differentiate into the decidualized phenotype
Conclusions
Human reproduction, though fundamental to our species survival, remains strikingly inefficient. Reproduction of an organism is viewed as if it is something that organisms have full control of, as if an organism makes copies of itself, by itself. Strictly speaking, such language is appropriate only to asexually reproducing populations since, as sexually reproducing organisms neither produce clones, nor produce other organisms by themselves. The probability of conception during one menstrual cycle is approximately 30% [75]. Accordingly, a better understanding of the molecular mechanisms responsible for implantation will improve treatment for infertility and early pregnancy loss in women.
Differentiation of a stromal fibroblast to a decidualized cell is essential for the maintenance of pregnancy in the primate. As the primate endometrium undergoes cyclical changes during menstrual cycle in preparation for implantation, an embryonic “signal” is responsible for rescuing stromal fibroblasts from apoptosis and normal regression at the end of each non-pregnant ovarian cycle. Notch signaling dictates cell fate and critically influences cell proliferation, differentiation, and apoptosis. Seemingly, the co-expression of αSMA and Notch1, both arising from CG signaling, inhibits apoptosis of stromal cells during the establishment of pregnancy. Low expression of Notch1 can be correlated to shedding of the uterine lining and the inability of the uterus to accept an embryo. Supplementation with (h)CG or progesterone will induce Notch1 expression to mediate the survival of the uterine lining.
Acknowledgement
This work was supported by NIH HD 42280.
Footnotes
Chorionic gonadotropin and Notch1 inhibit stromal cell apoptosis and induce decidualization in the primate endometrium.
References
- 1.Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril. 2004;81:1265–1269. doi: 10.1016/j.fertnstert.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 2.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 3.Roberts CaLC. Where have all the conceptions gone? Lancet. 1975;1:636–637. [PubMed] [Google Scholar]
- 4.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ\ 1997;315:32–34. doi: 10.1136/bmj.315.7099.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–584. doi: 10.1016/S0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- 8.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, et al. Identification of novel genes regulated by chorionic gonadotropin n baboon endometrium during the window of implantation. Endocrinology. 2007;148:618–626. doi: 10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- 10.Filicori M, Fazleabas AT, Huhtaniemi I, Licht P, Rao Ch V, Tesarik J, et al. Novel concepts of human chorionic gonadotropin: reproductive system interactions and potential in the management of infertility. Fertil Steril. 2005;84:275–284. doi: 10.1016/j.fertnstert.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Cameo P, Szmidt M, Strakova Z, Mavrogianis P, Sharpe-Timms KL, Fazleabas AT. Decidualization regulates the expression of the endometrial chorionic gonadotropin receptor in the primate. Biol Reprod. 2006;75:681–689. doi: 10.1095/biolreprod.106.051805. [DOI] [PubMed] [Google Scholar]
- 12.Enders A. Overview of the morphology of implantation in primates. New York: Springer; 1993. [Google Scholar]
- 13.Ghosh D, Sengupta J. Recent developments in endocrinology and paracrinology of blastocyst implantation in the primate. Hum Reprod Updat. 1998;4:153–168. doi: 10.1093/humupd/4.2.153. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101:17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/me.15.1.184. [DOI] [PubMed] [Google Scholar]
- 16.Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA. 1999;96:2543–2548. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, et al. Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science. 1989;245:494–499. doi: 10.1126/science.2502842. [DOI] [PubMed] [Google Scholar]
- 18.Rousseau-Merck MF, Misrahi M, Atger M, Loosfelt H, Milgrom E, Berger R. Localization of the human luteinizing hormone/choriogonadotropin receptor gene (LHCGR) to chromosome 2p21. Cytogenet Cell Genet. 1990;54:77–79. doi: 10.1159/000132962. [DOI] [PubMed] [Google Scholar]
- 19.Segaloff DL, Ascoli M. The lutropin/choriogonadotropin receptor ... 4 years later. Endocr Rev. 1993;14:324–347. doi: 10.1210/er.14.3.324. [DOI] [PubMed] [Google Scholar]
- 20.Latronico AC, Segaloff DL. Naturally occurring mutations of the luteinizing-hormone receptor: lessons learned about reproductive physiology and G protein-coupled receptors. Am J Hum Genet. 1999;65:949–958. doi: 10.1086/302602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefkowitz RJ, Hausdorff WP, Caron MG. Role of phosphorylation in desensitization of the beta-adrenoceptor. Trends Pharmacol Sci. 1990;11:190–194. doi: 10.1016/0165-6147(90)90113-M. [DOI] [PubMed] [Google Scholar]
- 22.Cameo P, Szmidt M, Strakova Z, Mavrogianis P, Sharpe-Timms KL, Fazleabas AT. Decidualization regulates the expression of the endometrial chorionic gonadotrophin receptor in the primate. Biol Reprod. 2006;75:681–689. doi: 10.1095/biolreprod.106.051805. [DOI] [PubMed] [Google Scholar]
- 23.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 24.Weinmaster G, Roberts VJ, Lemke G. A homolog of Drosophila Notch expressed during mammalian development. Development. 1991;113:199–205. doi: 10.1242/dev.113.1.199. [DOI] [PubMed] [Google Scholar]
- 25.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 26.Weinmaster G. The ins and outs of notch signaling. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- 27.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 28.Artavanis-Tsakonas S, Simpson P. Choosing a cell fate: a view from the Notch locus. Trends Genet. 1991;7:403–408. doi: 10.1016/0168-9525(91)90264-q. [DOI] [PubMed] [Google Scholar]
- 29.Jang MS, Zlobin A, Kast WM, Miele L. Notch signaling as a target in multimodality cancer therapy. Curr Opin Mol Ther. 2000;2:55–65. [PubMed] [Google Scholar]
- 30.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-N. [DOI] [PubMed] [Google Scholar]
- 32.Jen WC, Wettstein D, Turner D, Chitnis A, Kintner C. The Notch ligand, X-Delta-2, mediates segmentation of the paraxial mesoderm in Xenopus embryos. Development. 1997;124:1169–1178. doi: 10.1242/dev.124.6.1169. [DOI] [PubMed] [Google Scholar]
- 33.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 34.Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. doi: 10.1016/S0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 35.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci USA. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/S1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 37.Kidd S, Kelley MR, Young MW. Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol. 1986;6:3094–3108. doi: 10.1128/mcb.6.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985;43:567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K, Taniguchi Y, Minoguchi S, Sakai T, Tun T, Furukawa T, et al. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J kappa/Su(H) Curr Biol. 1995;5:1416–1423. doi: 10.1016/S0960-9822(95)00279-X. [DOI] [PubMed] [Google Scholar]
- 40.Rechsteiner M. Regulation of enzyme levels by proteolysis: the role of pest regions. Adv Enzyme Regul. 1988;27:135–151. doi: 10.1016/0065-2571(88)90015-5. [DOI] [PubMed] [Google Scholar]
- 41.Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 42.Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, et al. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- 43.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swiatek PJ, Lindsell CE, Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 45.Kopan R, Weintraub H. Mouse notch: expression in hair follicles correlates with cell fate determination. J Cell Biol. 1993;121:631–641. doi: 10.1083/jcb.121.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conlon RA, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 47.Psychoyos A. Endocrine control of egg implantation. Washington, DC: American Physiological Society; 1973. [Google Scholar]
- 48.Banaszak S, Brudney A, Donnelly K, Chai D, Chwalisz K, Fazleabas AT. Modulation of the action of chorionic gonadotropin in the baboon (Papio anubis) uterus by a progesterone receptor antagonist (ZK 137. 316) Biol Reprod. 2000;63:820–825. doi: 10.1095/biolreprod63.3.820. [DOI] [PubMed] [Google Scholar]
- 49.Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod. 1998;59:160–168. doi: 10.1095/biolreprod59.1.160. [DOI] [PubMed] [Google Scholar]
- 50.Shelly LL, Fuchs C, Miele L. Notch-1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. J Cell Biochem. 1999;73:164–175. doi: 10.1002/(SICI)1097-4644(19990501)73:2<164::AID-JCB3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- 52.Jang MS, Miao H, Carlesso N, Shelly L, Zlobin A, Darack N, et al. Notch-1 regulates cell death independently of differentiation in murine erythroleukemia cells through multiple apoptosis and cell cycle pathways. J Cell Physiol. 2004;199:418–433. doi: 10.1002/jcp.10467. [DOI] [PubMed] [Google Scholar]
- 53.Falco M, Cobellis L, Giraldi D, Mastrogiacomo A, Perna A, Colacurci N, et al. Expression and distribution of notch protein members in human placenta throughout pregnancy. Placenta. 2007;28:118–126. doi: 10.1016/j.placenta.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167:4458–4467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 55.Nakazawa M, Ishii H, Nakamura H, Yoshino SI, Fukamizu A, Nishioka K, et al. NFkappaB2 (p52) promoter activation via Notch signaling pathway in rheumatoid synoviocytes. Int J Mol Med. 2001;7:31–35. doi: 10.3892/ijmm.7.1.31. [DOI] [PubMed] [Google Scholar]
- 56.Hopwood D, Levison DA. Atrophy and apoptosis in the cyclical human endometrium. J Pathol. 1976;119:159–166. doi: 10.1002/path.1711190305. [DOI] [PubMed] [Google Scholar]
- 57.Tabibzadeh S, Zupi E, Babaknia A, Liu R, Marconi D, Romanini C. Site and menstrual cycle-dependent expression of proteins of the tumour necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production of TNF alpha in human endometrium. Hum Reprod. 1995;10:277–286. doi: 10.1093/oxfordjournals.humrep.a135928. [DOI] [PubMed] [Google Scholar]
- 58.Vaskivuo TE, Stenback F, Karhumaa P, Risteli J, Dunkel L, Tapanainen JS. Apoptosis and apoptosis-related proteins in human endometrium. Mol Cell Endocrinol. 2000;165:75–83. doi: 10.1016/S0303-7207(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 59.Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab. 2005;90:2351–2356. doi: 10.1210/jc.2004-2130. [DOI] [PubMed] [Google Scholar]
- 60.Mashima T, Naito M, Tsuruo T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene. 1999;18:2423–2430. doi: 10.1038/sj.onc.1202558. [DOI] [PubMed] [Google Scholar]
- 61.Suarez-Huerta N, Lecocq R, Mosselmans R, Galand P, Dumont JE, Robaye B. Myosin heavy chain degradation during apoptosis in endothelial cells. Cell Prolif. 2000;33:101–114. doi: 10.1046/j.1365-2184.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christensen S, Verhage HG, Nowak G, Lanerolle P, Fleming S, Bell SC, et al. Smooth muscle myosin II and alpha smooth muscle actin expression in the baboon (Papio anubis) uterus is associated with glandular secretory activity and stromal cell transformation. Biol Reprod. 1995;53:598–608. doi: 10.1095/biolreprod53.3.598. [DOI] [PubMed] [Google Scholar]
- 63.Kim JJ, Wang J, Bambra C, Das SK, Dey SK, Fazleabas AT. Expression of cyclooxygenase-1 and -2 in the baboon endometrium during the menstrual cycle and pregnancy. Endocrinology. 1999;140:2672–2678. doi: 10.1210/en.140.6.2672. [DOI] [PubMed] [Google Scholar]
- 64.Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, et al. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005;146:4097–4104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- 65.Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology. 1999;140:997–1004. doi: 10.1210/en.140.2.997. [DOI] [PubMed] [Google Scholar]
- 66.Alvarado MV, Alvarado NE, Russo J, Russo IH. Human chorionic gonadotropin inhibits proliferation and induces expression of inhibin in human breast epithelial cells in vitro. In Vitro Cell Dev Biol Anim. 1994;30A:4–8. doi: 10.1007/BF02631407. [DOI] [PubMed] [Google Scholar]
- 67.Han SW, Lei ZM, Rao CV. Up-regulation of cyclooxygenase-2 gene expression by chorionic gonadotropin during the differentiation of human endometrial stromal cells into decidua. Endocrinology. 1996;137:1791–1797. doi: 10.1210/en.137.5.1791. [DOI] [PubMed] [Google Scholar]
- 68.Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, et al. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94:910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- 69.Noseda M, Fu Y, Niessen K, Wong F, Chang L, McLean G, et al. Smooth muscle alpha-actin is a direct target of Notch/CSL. Circ Res. 2006;98:1468–1470. doi: 10.1161/01.RES.0000229683.81357.26. [DOI] [PubMed] [Google Scholar]
- 70.Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147:4112–4121. doi: 10.1210/en.2005-1577. [DOI] [PubMed] [Google Scholar]
- 71.Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, Velde ER, et al. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab. 2001;86:3421–3429. doi: 10.1210/jc.86.7.3421. [DOI] [PubMed] [Google Scholar]
- 72.Vaskivuo TE, Stenback F, Tapanainen JS. Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer. 2002;95:1463–1471. doi: 10.1002/cncr.10876. [DOI] [PubMed] [Google Scholar]
- 73.Cameo P, Srisuparp S, Strakova Z, Fazleabas AT. Chorionic gonadotropin and uterine dialogue in the primate. Reprod Biol Endocrinol. 2004;2:50. doi: 10.1186/1477-7827-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotrophin and decidualization in vitro inhibits cytochalasin D induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147:4112–4121. doi: 10.1210/en.2005-1577. [DOI] [PubMed] [Google Scholar]
- 75.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]