Abstract
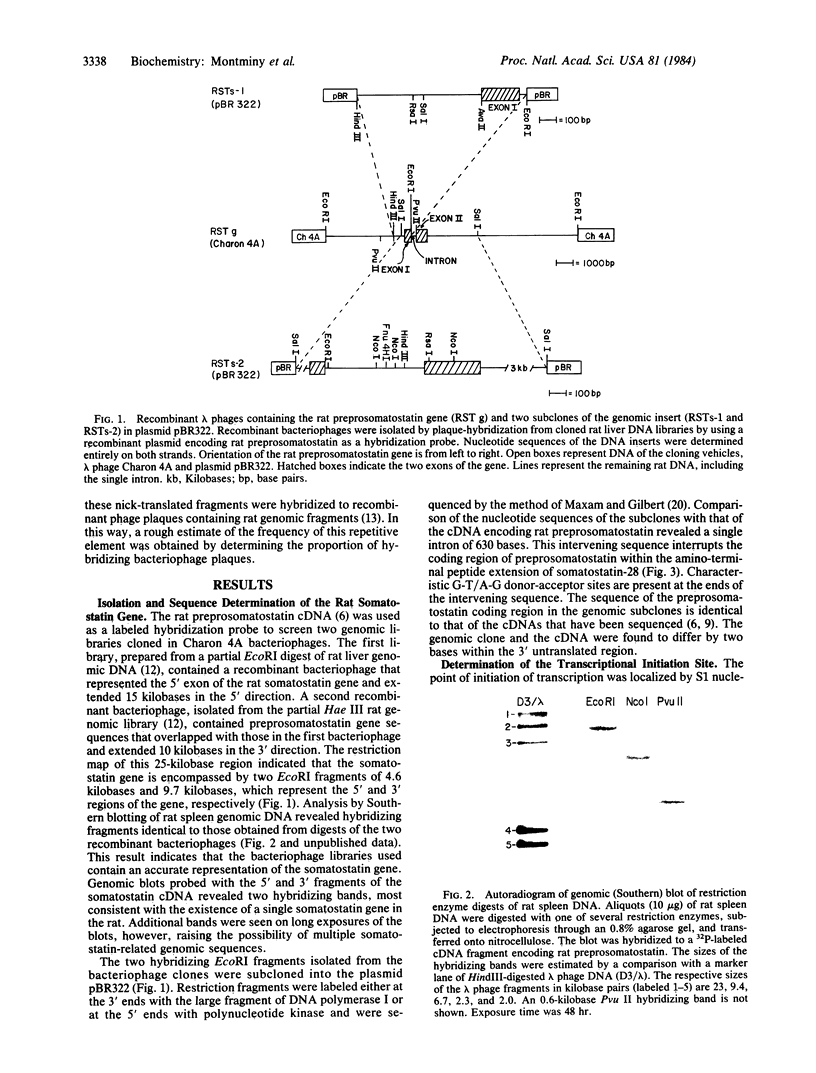
The somatostatins are peptides of 14 and 28 amino acids that are produced in a variety of endocrine and nonendocrine tissues. These peptides inhibit the secretion of many different pituitary, pancreatic, and gastrointestinal hormones. Previously, we have reported the isolation and nucleotide sequence of a cDNA derived from a rat medullary thyroid carcinoma that encoded preprosomatostatin , a 116-amino-acid precursor of somatostatin. We now report the structural characterization of the rat somatostatin gene isolated from recombinant bacteriophage libraries prepared from rat liver DNA. The gene spans 1.2 kilobases and is interrupted within the coding sequence of prosomatostatin by a single intron of 630 bases. A sequence characteristic of a Goldberg- Hogness promoter ("TATA" box), T-T-T-A-A-A-A, is located 31 bases upstream from the transcriptional initiation site. A repetitive DNA sequence, highly reiterated in the rat genome, is located in the 5' flanking region of the gene within 900 bases of the initiation site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron D. C., Muszynski M., Birnbaum R. S., Sabo S. W., Roos B. A. Somatostatin elaboration by monolayer cell cultures derived from transplantable rat medullary thyroid carcinoma: synergistic stimulatory effects of glucagon and calcium. Endocrinology. 1981 Dec;109(6):1830–1834. doi: 10.1210/endo-109-6-1830. [DOI] [PubMed] [Google Scholar]
- Benoit R., Böhlen P., Ling N., Briskin A., Esch F., Brazeau P., Ying S. Y., Guillemin R. Presence of somatostatin-28-(1-12) in hypothalamus and pancreas. Proc Natl Acad Sci U S A. 1982 Feb;79(3):917–921. doi: 10.1073/pnas.79.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R., Ling N., Alford B., Guillemin R. Seven peptides derived from pro-somatostatin in rat brain. Biochem Biophys Res Commun. 1982 Aug;107(3):944–950. doi: 10.1016/0006-291x(82)90614-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Funckes C. L., Minth C. D., Deschenes R., Magazin M., Tavianini M. A., Sheets M., Collier K., Weith H. L., Aron D. C., Roos B. A. Cloning and characterization of a mRNA-encoding rat preprosomatostatin. J Biol Chem. 1983 Jul 25;258(14):8781–8787. [PubMed] [Google Scholar]
- Gerich J. E., Patton G. S. Somatostatin. Physiology and clinical applications. Med Clin North Am. 1978 Mar;62(2):375–392. doi: 10.1016/s0025-7125(16)31813-2. [DOI] [PubMed] [Google Scholar]
- Goodman R. H., Aron D. C., Roos B. A. Rat pre-prosomatostatin. Structure and processing by microsomal membranes. J Biol Chem. 1983 May 10;258(9):5570–5573. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Lechan R. M., Goodman R. H., Rosenblatt M., Reichlin S., Habener J. F. Prosomatostatin-specific antigen in rat brain: localization by immunocytochemical staining with an antiserum to a synthetic sequence of preprosomatostatin. Proc Natl Acad Sci U S A. 1983 May;80(9):2780–2784. doi: 10.1073/pnas.80.9.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Y. C., Wheatley T., Ning C. Multiple forms of immunoreactive somatostatin: comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology. 1981 Dec;109(6):1943–1949. doi: 10.1210/endo-109-6-1943. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983 Dec 15;309(24):1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. P., Pictet R. L., Rutter W. J. Human somatostatin I: sequence of the cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4575–4579. doi: 10.1073/pnas.79.15.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Trent D. F., Weir G. C. Heterogeneity of somatostatin-like peptides in rat brain, pancreas, and gastrointestinal tract. Endocrinology. 1981 Jun;108(6):2033–2038. doi: 10.1210/endo-108-6-2033. [DOI] [PubMed] [Google Scholar]
- Uhler M., Herbert E., D'Eustachio P., Ruddle F. D. The mouse genome contains two nonallelic pro-opiomelanocortin genes. J Biol Chem. 1983 Aug 10;258(15):9444–9453. [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]