Abstract
Purpose
To assess the expression of PTEN and total and phosphorylated Akt in human ovarian follicles during follicular growth.
Methods
Immunohistochemistry of ovarian tissues and Western blotting and immunofluorescence of primary cultured luteinized granulosa cells for PTEN and Akt.
Results
Immunoreactivity of Akt was found in the oocytes, granulosa cells and theca cells in primordial follicles, follicles at each growing stage and luteal cells. As the follicles grew, staining for PTEN became intense in the granulosa cells, whereas the intensity of phospho-Akt became weak. Western blotting and immunofluorescence analysis using primary cultured granulosa-lutein cells showed Akt and PTEN expression, and phosphorylation of Akt in vitro.
Conclusions
PTEN and Akt are present in the granulosa cells during folliculogenesis. An increase in PTEN may lead to changes in proliferation and/or differentiation of granulosa cells during follicular growth via regulation of Akt phosphorylation.
Keywords: Akt, Folliculogenesis, Granulosa cells, Phosphorylation, PTEN
Introduction
The molecular reprogramming that leads to oocyte maturation is under the regulatory control of signals from the proliferating granulosa cells, in response to the stimulation of gonadotropin and growth factors [1]. In addition, during terminal follicular growth and initial luteinization, it is necessary that the transition of granulosa cells to a less proliferative and highly steroidogenic form occurs in response to luteinizing hormone (LH).
Among growth factors that stimulate granulosa cell proliferation, several studies have shown insulin-like growth factor (IGF)-I is involved in the survival of granulosa cells in humans [2, 3] and other species [4–6]. The biological functions of IGF-1 are mainly mediated by the type-I IGF receptor (IGF-IR) that is able to activate the phosphatidylinositol 3-kinase (PI3K)/Akt pathway [7]. PI3K generates phosphatidylinositol-3,4,5-triphosphate (PIP3), which activates multiple downstream effectors including Akt, by phosphorylating phosphatidylinositol-4,5-biphosphate (PIP2) [8]. Such activation is correlated with cell survival in a wide variety of cells, including those of epithelial, mesenchymal and neuronal origin [9, 10], as well as cancer cells [11]. In bovine granulosa cells, Akt, a downstream substrate of PI3K, was necessary for the protective effect of IGF-1 against FasL-induced apoptosis [12]. Akt has been also demonstrated to be involved in granulosa cell survival in hens [6] and pigs [13]. However, regulation of expression and phosphorylation of Akt in each stage of follicle development has not been evaluated, especially in humans, although activation of Akt under stimulation of IGF-1 is well known to be necessary for proliferation of granulosa cells.
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) lipid phosphatase antagonizes the activity of PI3K by dephosphorylating PIP3 to PIP2 [14]. Therefore, PTEN indirectly inhibits Akt phosphorylation and its activation or inactivation results in decreased or increased Akt activity [15]. Originally, PTEN was reported to be a tumor suppressor gene frequently inactivated in a number of cancers including prostate, brain and endometrial cancer. Recently, alterations in the expression and activity of PTEN have been proposed to play a causal role in the development of several conditions other than cancer, including rheumatoid arthritis, chronic obstructive pulmonary disease and pulmonary fibrosis [16, 17]. It is not fully understood how PTEN expression is regulated [18]. However, the balance between PI3K and PTEN determines PIP3 levels, which affect a variety of intracellular signals, such as Akt. In human ovaries, PTEN and the PI3K/Akt pathway might be involved in the regulation of proliferation and differentiation of granulosa cells, although expression of PTEN in human ovaries has hardly been investigated. Therefore, the objectives of the present study were to identify and characterize expression of Akt and PTEN in each stage of follicle development in human ovaries and assess the relationship between activation of Akt and PTEN expression in the same follicles in vivo. We also investigated expression of Akt and PTEN, and phosphorylation of Akt in human granulosa cells in vitro.
Materials and methods
Tissue collection
We retrieved normal ovary specimens from the pathology files at Nagoya University Hospital as described previously [19]. Briefly, cases were selected on the basis of a history of regular menstrual cycles and no use of any intrauterine device or hormone therapy for ≥ 6 months before surgery. Following these reviews, a portion of whole ovarian tissue (n = 52) was obtained from women undergoing surgical procedures for a variety of benign gynecological conditions. On the basis of endometrial histological dating and the patient’s last menstrual period, the luteal tissues were from the early, middle and the late phase of the luteal phase. These samples were fixed in 10% formalin, embedded in paraffin, and used for immunohistochemistry.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections were cut to a thickness of 3 μm. Deparaffinized sections in 0.01 M citrate buffer were treated three times for 5 min at 90°C at 750 W in an H2500 microwave oven (M&M, Tokyo, Japan) for heat-induced epitope retrieval. Endogenous peroxidase activity was blocked by incubation with 0.5% (w/v) hydrogen peroxide in methanol for 10 min, and nonspecific immunoglobulin binding was blocked by incubation with 10% normal goat serum in phosphate-buffered saline (PBS) for 10 min. Then, specimens were incubated with the primary antibody against human Akt (H-136, a rabbit polyclonal antibody; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:100, human PTEN (A2B1, a mouse monoclonal antibody; Santa Cruz Biotechnology, Inc.) diluted 1:100 or human phospho-Akt (587F11, a mouse monoclonal antibody; Cell Signaling Technology, Inc., Danvers, MA) diluted 1:200 in PBS at 4°C overnight in a humidified chamber. After incubation, the specimens were washed twice in PBS and then incubated at room temperature for 10 min with a biotin-conjugated secondary antibody. Immunohistochemical staining was performed on the basis of the labeled streptavidin-biotin method (Histofine; Nichirei Bioscience, Inc., Tokyo, Japan). A Ventana Basic DAB Detection Kit (Ventana Medical Systems, Tucson, AZ), yielding a brown product form of diaminobenzidine (DAB)-copper sulfate, was used to detect each protein. In the negative control experiments, the primary antibody was replaced with rabbit or mouse IgG. The slides were counterstained with hematoxylin before mounting. Stained sections were observed under a BH2 microscope (Olympus, Tokyo, Japan) and photographed using a CCD Color Camera CS600 (Olympus). Evaluation of the staining intensity was undertaken with the criteria as follows: negative, occasional or weak, moderate, or strong.
Isolation and culture of luteinized granulosa cells and IGF-1 stimulation
Human luteinized granulosa cells were collected from follicular fluid obtained via ultrasound-guided transvaginal oocyte retrieval. The luteinized granulosa cells after isolation of the oocytes were separated from red blood cells with Percoll gradient (Amersham Biosciences Corp., Piscataway, NJ) and resuspended in DMEM (Sigma, St. Louis, MO) containing 10% FCS (Sigma), 100 IU/ml penicillin, 100 μg/ml streptomycin, 25 mg/l amphotericin and l-glutamine. Then luteinized granulosa cells were seeded onto a 60-mm sterile collagen-coated dish (Biocoat; Becton Dickinson and Co., Franklin Lakes, NJ) or a four-well chamber slide (Lab-Tek Chamber Slide; Nalge Nunc International, Rochester, NY). The luteinized granulosa cells were stimulated with 100 ng/ml IGF-1 (Sigma) for 10 min after overnight serum starvation.
Western blotting
Primary cultured luteinized granulosa cells were lysed in RIPA buffer (10 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 5 mM EDTA, 1% sodium deoxycholate, 0.1% SDS, 1.2% aprotinin, 5 μM leupeptine, 4 μM antipain, 1 mM phenylmethylsulfonylfluoride and 0.1 mM Na3VO4). Cell lysates were clarified by centrifugation at 13,000×g at 4°C for 15 min and diluted in a 2× sample buffer (125 mM Tris–HCl, pH 6.8, 4% SDS, 10% glycerol, 0.2% bromphenolblue and 4% 2-mercaptoethanol). The protein extract (10 μg) was separated by 10% SDS-PAGE, and transferred to a nitrocellulose membrane. After blocking in PBS containing 5% skim milk for 1 h, the membranes were immunoblotted with anti-human Akt Ab (H-136; 1:1,000), anti-human PTEN Ab (A2B1; 1:1,000) or anti-human phospho-Akt Ab (587F11; 1:500). Immunoreactive proteins were stained using an enhanced chemiluminescence system (ECL; Amersham Biosciences).
Immunofluorescence
Primary cultured luteinized granulosa cells after fixation with 3.7% formaldehyde in PBS were treated with 0.2% Triton-X-100 in PBS for 10 min and soaked in PBS containing 1% BSA (BSA-PBS) and 5% skim milk for 30 min. Cells were then incubated with anti-human Akt Ab (H-136; 1:100) or anti-human PTEN Ab (A2B1; 1:100) for 1 h, washed three times for 5 min with BSA-PBS, and incubated with secondary antibodies rhodamine-conjugated anti-rabbit IgG and fluorescein isothiocyanate-conjugated anti-mouse IgG (Chemicon International, Inc., Temecula, CA) for 1 h. In the negative control experiments, the primary antibody was replaced with rabbit or mouse IgG. Cells were examined using a BZ-8000 fluorescent microscope (Keyence Corporation, Osaka, Japan).
Results
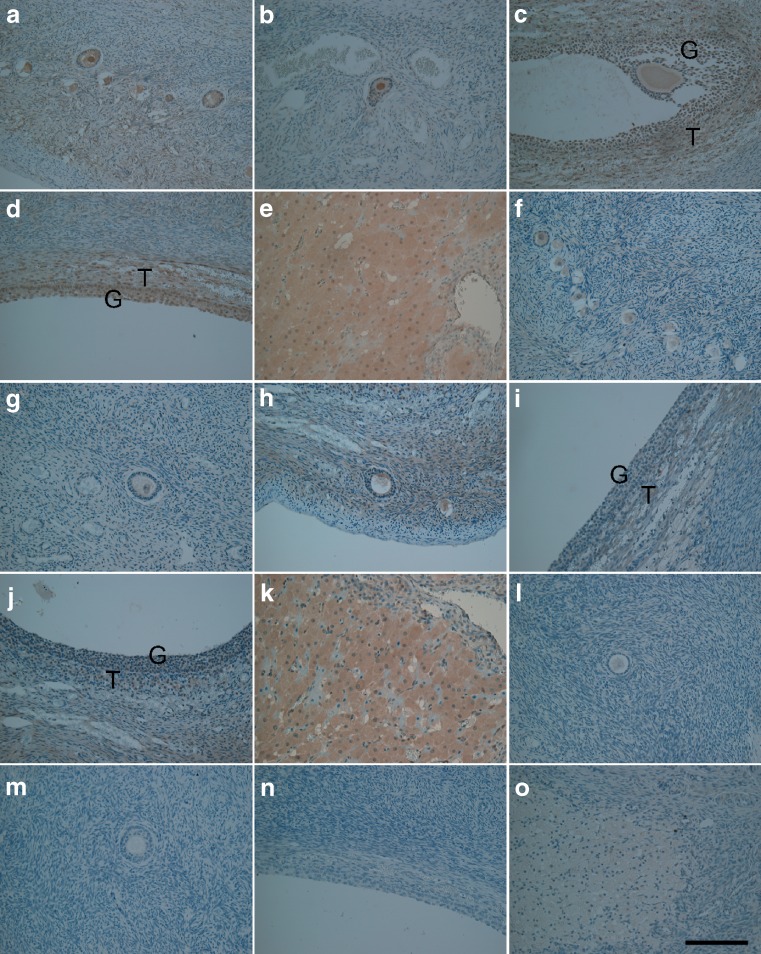
Immunoreactivity of Akt was found in the oocytes, granulosa cells and theca cells in primordial follicles and follicles at each growing stage (Fig. 1a–d). Akt was also expressed in the luteal cells in the mid-luteal phase (Fig. 1e). The staining intensity was moderate in the granulosa cells in the primary follicles, whereas the granulosa cells in the antral and preovulatory follicles were strongly stained. Weak and occasional staining for PTEN occurred in the granulosa cells in the primary and antral follicles (Fig. 1f–i). The granulosa cells and theca cells in the preovulatory follicles displayed moderate staining (Fig. 1j). The luteal cells were strongly stained in the mid-luteal phase, similar to Akt staining (Fig. 1k). Immunohistochemistry shown here are the representative ones which applied to most of samples. Both Akt and PTEN exhibited a distinct distribution and variation of the staining intensity depending on the cell type, as well as follicular development (Table 1).
Fig. 1.
Immunohistochemical localization of Akt (a–e), PTEN (f–k) and negative controls using rabbit IgG (l, n) or mouse IgG (m, o) in human ovarian tissues displaying immunostaining with various intensities in association with primordial and primary follicles (a, b, f–h, l, m), secondary and preovulatory follicles (c, d, i, j, n) and luteal tissues from the mid-luteal phase (e, k, o). G Granulosa cells, T theca cells. Original magnification: ×200. Bar in (o) = 100 μm
Table 1.
Immunostaining of ovarian structures with specific antibodies for Akt and PTEN
| Akt | PTEN | |
|---|---|---|
| Primordial follicles | ||
| Oocytes | +a | +/− or + |
| Pregranulosa cells | + | – |
| Primary/Preantral follicles | ||
| Oocytes | + | +/− |
| Granulosa cells | + | +/− |
| Secondary/Antral follicles | ||
| Oocytes | + | +/− |
| Granulosa cells | ++ | +/− |
| Theca cells | + | +/− |
| Graafian/Preovulatory follicles | ||
| Oocytes | + | +/− |
| Granulosa cells | ++ | +/− or + |
| Theca cells | + | +/− or + |
| Corpus luteum (mid-luteal) | ||
| Large luteal cells | ++ | + |
aStaining intensity is expressed as − negative, +/− occasional or weak, + moderate, or ++ strong.
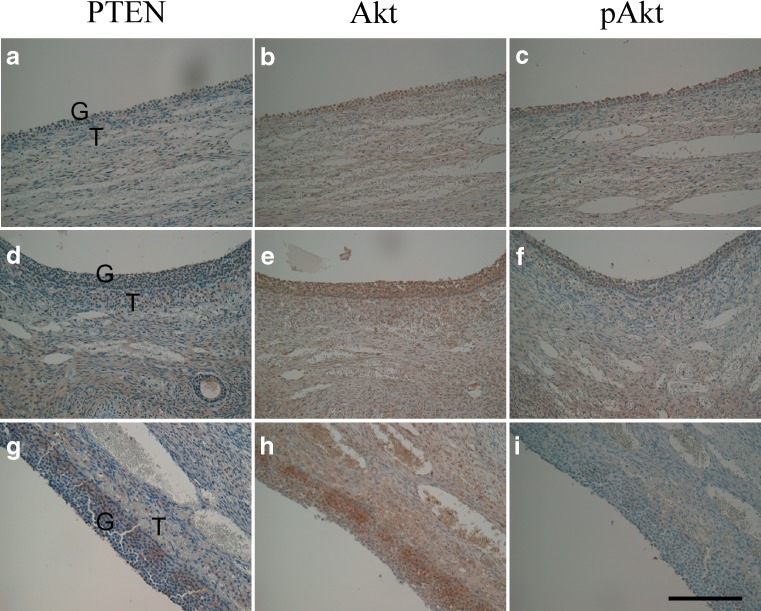
Immunohistochemistry for serial sections showed an inverse relationship between PTEN and phospho-Akt, i.e. the staining intensity of phospho-Akt in the granulosa cells was strong or moderate with negligible or weak expression of PTEN (Fig. 2a–f), whereas weak staining for phospho-Akt occurred in the granulosa cells, which stained moderately for PTEN (Fig. 2g–i).
Fig. 2.
Immunohistochemical localization of PTEN (a, d, g), Akt (b, e, h) and phospho-Akt (c, f, i) in the three sets of serial sections (a–c, d–f and g–i) displaying immunostaining in granulosa ant theca cells of preovulatory follicles. pAkt, phospho-Akt. G Granulosa cells, T theca cells. Original magnification: ×200. Bar in (i) = 100 μm
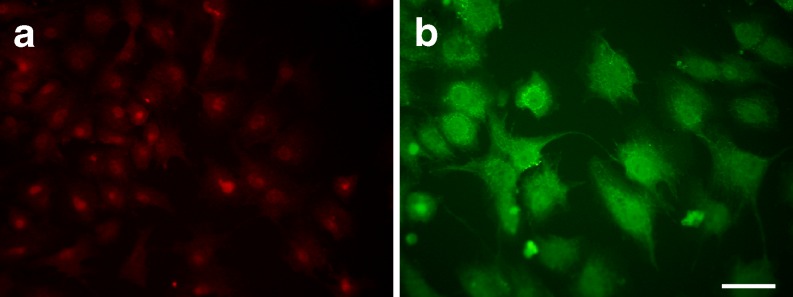
We next performed Western blotting and immunofluorescence analysis using primary cultured luteinized granulosa cells to confirm the expression of Akt and PTEN, and phosphorylation of Akt in vitro. Immunofluorescence analysis showed that both Akt (Fig. 3a) and PTEN (Fig. 3b) were expressed in the primary cultured luteinized granulosa cells obtained from IVF. Intense expression of PTEN was observed in the cytoplasm, while the cells showed nuclear and cytoplasmic localization with the Akt antibody. Western blotting showed that primary cultured luteinized granulosa cells expressed PTEN and Akt, which was phosphorylated by IGF–1 (Fig. 4).
Fig. 3.
Expression of Akt and PTEN in the primary cultured luteinized granulosa cells obtained from IVF by immunofluorescence. a The cells showed nuclear and cytoplasmic localization of Akt. b Intense expression of PTEN was observed in the cytoplasm of the cells. Bar in (b) = 20 μm.
Fig. 4.
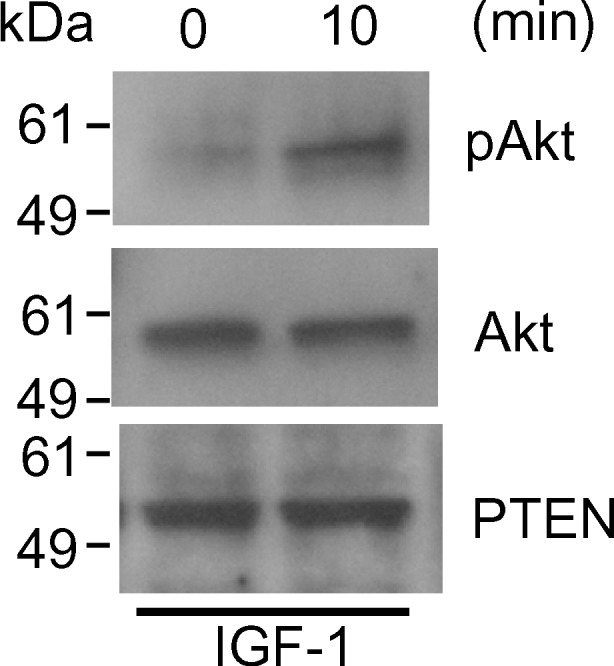
Expression of Akt and PTEN, and phosphorylation of Akt in primary cultured luteinized granulosa cells with 100 ng/ml IGF-1 for 10 min. Cells were lysed, subjected to SDS-PAGE (10 μg per lane), and immunoblotted using anti-phospho-Akt (pAkt) Ab (top), anti-Akt Ab (middle), and anti-PTEN Ab (bottom). The phosphorylation of Akt was induced by IGF-1 in luteinized granulosa cells. Experiments were repeated three times with similar results
Discussion
Granulosa cells proliferate and then undergo differentiation, an inverse relationship between both processes being observed during terminal follicular growth [20]. We found constant expression of Akt in granulosa cells and oocytes in follicles at each growing stage. On the other hand, granulosa cells in large preovulatory follicles were shown to express higher levels of PTEN than granulosa cells in small follicles showing negligible or occasional staining, suggesting that PTEN expression increases during terminal follicular growth. Immunofluorescent analysis and Western blotting showed obvious expression of PTEN in luteinized granulosa cells. Immunohistochemistry for Akt, pAkt and PTEN in serial sections showed good agreement with the function of PTEN, which dephosphorylates Akt, i.e. immunohistochemistry for serial sections showed an inverse relationship between PTEN and phospho-Akt. PTEN phosphatase antagonizes the activity of PI3K that phosphorylates Akt. It has been demonstrated that Fas-mediated apoptosis is impaired in PTEN-mutant mice, in which phosphorylation of Akt is abnormally elevated [21]. All things considered, our results suggest the possible involvement of PTEN, which increases in expression during terminal follicular development and luteinization, in the regulation of proliferation and differentiation of granulosa cells via regulation of Akt phosphorylation.
We also found occasional expression of PTEN in small follicles, which might affect follicular development through dephosphorylation/inactivation of Akt. Overexpression of PTEN in vivo and vitro in different cell lines induces the arrest of the cell cycle and/or apoptosis [22–24]. Taken together, expression of PTEN in granulosa cells in rather small follicles, which decrease the potential for proliferation as an inappropriate differentiation signal, might lead to apoptosis of granulosa cells followed by follicle atresia, although further studies are needed to investigate whether PTEN is implicated in the apoptosis of granulosa cells.
Stimulation with LH of matured preovulatory follicles induces the differentiation of granulosa cells to luteinized granulosa cells [25]. In addition, it has been demonstrated that several molecules, which are up-regulated with LH stimulation, are involved in granulosa cell differentiation and luteinization. Regulatory mechanism of PTEN expression even in other cells has not been well investigated. It may be useful how PTEN expression is regulated during terminal follicular growth to reveal the mechanism in differentiation and luteinization of granulosa cells.
Recently, Froment et al. [26] has demonstrated that PTEN increases during terminal follicular growth in ovine granulosa cells. To the best of our knowledge, this is the only published manuscript demonstrating PTEN expression in human granulosa cells.
In conclusion, the immunolocalization of Akt, pAkt and PTEN were studied in human ovaries and primary cultured luteinized granulosa cells. We report the expression of PTEN in the human ovaries for the first time. Our data suggest that PTEN expression in granulosa cells increases during proliferation/differentiation transition, which affect Akt phosphorylation. Interestingly, such an increase in PTEN expression has been shown in the proliferation/differentiation transition of human endometrial cells [27]. Further studies, such as cell proliferation assay, in vitro and in vivo are required to give a functional evaluation of PTEN in human ovaries and the mechanism of PTEN induction in proliferation/differentiation transition in granulosa cells.
Footnotes
Capsule
An increase in PTEN expression of human ovaries may lead to regulation of proliferation and differentation of granulosa cell via a decrease in phosphorylated Akt.
References
- 1.Moor RM, Dai Y, Lee C, Fulka J., Jr. Oocyte maturation and embryonic failure. Hum Reprod Updat. 1998;4:223–236. doi: 10.1093/humupd/4.3.223. [DOI] [PubMed] [Google Scholar]
- 2.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/er.20.4.535. [DOI] [PubMed] [Google Scholar]
- 3.Bencomo E, Perez R, Arteaga MF, Acosta E, Pena O, Lopez L, et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril. 2006;85:474–480. doi: 10.1016/j.fertnstert.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci. 2004;82(E-Suppl):E40–E52. doi: 10.2527/2004.8213_supplE40x. [DOI] [PubMed] [Google Scholar]
- 5.Guthrie HD, Garrett WM, Cooper BS. Follicle-stimulating hormone and insulin-like growth factor-I attenuate apoptosis in cultured porcine granulosa cells. Biol Reprod. 1998;58:390–396. doi: 10.1095/biolreprod58.2.390. [DOI] [PubMed] [Google Scholar]
- 6.Johnson AL, Bridgham JT, Swenson JA. Activation of the Akt/protein kinase B signaling pathway is associated with granulosa cell survival. Biol Reprod. 2001;64:1566–1574. doi: 10.1095/biolreprod64.5.1566. [DOI] [PubMed] [Google Scholar]
- 7.Roberts CT., Jr. Control of insulin-like growth factor (IGF) action by regulation of IGF-I receptor expression. Endocr J. 1996;43(Suppl):S49–S55. doi: 10.1507/endocrj.43.suppl_s49. [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 11.Sumitomo M, Iwase A, Zheng R, Navarro D, Kaminetzky D, Shen R, et al. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell. 2004;5:67–78. doi: 10.1016/S1535-6108(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 12.Hu CL, Cowan RG, Harman RM, Quirk SM. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Mol Endocrinol. 2004;18:326–338. doi: 10.1210/me.2003-0178. [DOI] [PubMed] [Google Scholar]
- 13.Westfall SD, Hendry IR, Obholz KL, Rueda BR, Davis JS. Putative role of the phosphatidylinositol 3-kinase-Akt signaling pathway in the survival of granulosa cells. Endocrine. 2000;12:315–321. doi: 10.1385/ENDO:12:3:315. [DOI] [PubMed] [Google Scholar]
- 14.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 15.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci. 2003;24:71–76. doi: 10.1016/S0165-6147(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 17.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, et al. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med. 2003;168:436–442. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gericke A, Munson M, Ross AH. Regulation of the PTEN phosphatase. Gene. 2006;374:1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Harata T, Ando H, Iwase A, Nagasaka T, Mizutani S, Kikkawa F. Localization of angiotensin II, the AT1 receptor, angiotensin-converting enzyme, aminopeptidase A, adipocyte-derived leucine aminopeptidase, and vascular endothelial growth factor in the human ovary throughout the menstrual cycle. Fertil Steril. 2006;86:433–439. doi: 10.1016/j.fertnstert.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 20.Monniaux D, Pisselet C. Control of proliferation and differentiation of ovine granulosa cells by insulin-like growth factor-I and follicle-stimulating hormone in vitro. Biol Reprod. 1992;46:109–119. doi: 10.1095/biolreprod46.1.109. [DOI] [PubMed] [Google Scholar]
- 21.Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten +/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 22.Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10:599–604. doi: 10.1093/hmg/10.6.599. [DOI] [PubMed] [Google Scholar]
- 23.Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110:815–825. doi: 10.1172/JCI200213829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Dupont J, Yakar S, Karas M, LeRoith D. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene. 2004;23:786–794. doi: 10.1038/sj.onc.1207162. [DOI] [PubMed] [Google Scholar]
- 25.Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 26.Froment P, Bontoux M, Pisselet C, Monget P, Dupont J. PTEN expression in ovine granulosa cells increases during terminal follicular growth. FEBS Lett. 2005;579:2376–2382. doi: 10.1016/j.febslet.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85:2334–2338. doi: 10.1210/jc.85.6.2334. [DOI] [PubMed] [Google Scholar]