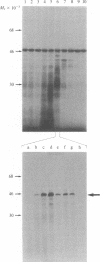
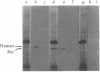
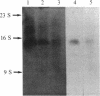
Abstract
We have cloned and identified a DNA sequence complementary to the mRNA of uroporphyrinogen decarboxylase ( UroDCase ) from rat. This mRNA is a minor species (0.1%) of the total mRNA from anemic rat spleen. Poly(A)+ mRNA was enriched for UroDCase mRNA to 20% purity by a very efficient procedure involving two successive steps of preparative gel electrophoresis under various denaturing conditions. cDNA prepared from partially purified UroDCase mRNA (1% purity) was cloned in the Pst I site of pBR322 by using the homopolymeric G-C tailing method. Primary screening of 500 clones from this cDNA library was performed with a cDNA probe complementary to highly purified mRNA for UroDCase (20% purity) and UroDCase cDNA clones were finally identified by hybrid-selected translation. The rat cDNA clones obtained hybridize to human UroDCase mRNA. This will permit the isolation of the corresponding human gene and molecular analysis of porphyria cutanea tarda, the commonest type of porphyria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Brûlet P., Roskam W. G. Improved method for cloning DNA complementary to minor mRNAs: preparation of a hybridization probe from purified mRNAs encoding intermediate filament proteins. Gene. 1983 Apr;22(1):59–68. doi: 10.1016/0378-1119(83)90064-1. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Rimkus D., Davidson N. Gel electrophoretic fractionation of RNAs by partial denaturation with methylmercuric hydroxide. Anal Biochem. 1979 Oct 15;99(1):200–206. doi: 10.1016/0003-2697(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Lee G. B., Tovey J. A. Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda. N Engl J Med. 1978 Aug 10;299(6):274–278. doi: 10.1056/NEJM197808102990603. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Sheppard D. M., Tovey J. A., Urquhart A. J. Immunoreactive uroporphyrinogen decarboxylase in porphyria cutanea tarda. Lancet. 1983 Jun 11;1(8337):1301–1304. doi: 10.1016/s0140-6736(83)92414-5. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Smith S. G., Herrero C., Lecha M., Mascaro J. M., Muniesa A. M., Czarnecki D. B., Brenan J., Poulos V., DE Salamanca R. E. Hepatoerythropoietic porphyria: a new uroporphyrinogen decarboxylase defect or homozygous porphyria cutanea tarda? Lancet. 1981 Apr 25;1(8226):916–919. doi: 10.1016/s0140-6736(81)91615-9. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Roméo P. H., de Verneuil H., Dubart A., Goossens M., Rosa J., Nordmann Y. Cell-free translation of human uroporphyrinogen decarboxylase mRNAs. Biochem Biophys Res Commun. 1984 Jan 13;118(1):378–382. doi: 10.1016/0006-291x(84)91112-4. [DOI] [PubMed] [Google Scholar]
- Granick J. L., Sassa S. Hemin control of heme biosynthesis in mouse Friend virus-transformed erythroleukemia cells in culture. J Biol Chem. 1978 Aug 10;253(15):5402–5406. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Itoh N., Nose K., Okamoto H. Purification and characterization of proinsulin mRNA from rat B-cell tumor. Eur J Biochem. 1979 Jun;97(1):1–9. doi: 10.1111/j.1432-1033.1979.tb13079.x. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Daegelen D., Dreyfus J. C. Cell-free translation of messenger RNAs from adult and fetal human muscle. Characterization of neosynthesized glycogen phosphorylase, phosphofructokinase and glucose phosphate isomerase. Eur J Biochem. 1981 May;116(1):7–12. doi: 10.1111/j.1432-1033.1981.tb05293.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. Porphyrin biosynthesis in erythrocytes. III. Uroporphyrinogen and its decarboxylase. J Biol Chem. 1958 Jun;232(2):1141–1162. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. Characterization of the homopolymer tailing reaction catalyzed by terminal deoxynucleotidyl transferase. Implications for the cloning of cDNA. J Biol Chem. 1982 Dec 25;257(24):14773–14782. [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E. The structure and protein kinase activity of proteins encoded by nonconditional mutants and back mutants in the sec gene of avian sarcoma virus. Virology. 1981 Jan 15;108(1):47–70. doi: 10.1016/0042-6822(81)90526-2. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. P., Besmond C., Cottreau D., Weber A., Chaumet-Riffaud P., Dreyfus J. C., Trépat J. S., Marie J., Kahn A. Molecular cloning of cDNA for rat L-type pyruvate kinase and aldolase B. J Biol Chem. 1983 Dec 10;258(23):14576–14584. [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Sassa S., Kappas A. Purification and properties of uroporphyrinogen decarboxylase from human erythrocytes. A single enzyme catalyzing the four sequential decarboxylations of uroporphyrinogens I and III. J Biol Chem. 1983 Feb 25;258(4):2454–2460. [PubMed] [Google Scholar]