Abstract
Androgen receptor (AR) is expressed throughout the osteoblast lineage. Two different AR transgenic families (AR2.3-tg and AR3.6-tg mice) demonstrating overlapping and distinct expression profiles were employed to assess the effects of enhanced androgen sensitivity to ameliorate hypogonadal loss. Two different paradigms of steroid replacement following orchidectomy (ORX) were used as either preventative or therapeutic therapy. ORX was performed in male wild-type (WT), AR2.3-tg and AR3.6-tg mice at 5 months with immediate DHT replacement (prevention, higher turnover) or at 3 months with DHT treatment delayed for 2 months (therapeutic, lower turnover), both with treatment for the last 6 weeks. Dual energy x-ray absorptiometry (DXA), microcomputed tomography (μCT), and histomorphometry were performed. In the prevention model, ORX significantly reduced BMD and BMC in all genotypes compared to sham and DHT was effective at prevention of osteopenia. In the therapeutic model, all genotypes became osteopenic compared to sham, but after a prolonged hypogonadal period, delayed DHT treatment provided little benefit. μCT analysis of mid-shaft total bone in all genotypes generally showed reductions after ORX. Delayed DHT was ineffective at restoring bone volume in any genotype whereas immediate treatment prevented loss only in AR transgenic mice. Cortical thickness also decreased with ORX but immediate DHT treatment was effective to increase thickness only in WT mice, likely due to expansion of marrow volume in both AR-tg lines. In metabolically highly active cancellous bone, ORX resulted in lower bone volume/tissue volume (BV/TV) in all genotypes, consistent among 3 sites measured. Again with delayed treatment, there was little effect of DHT to restore BV/TV, but when administered at the time of ORX, DHT completely prevented the decrease in cancellous bone in all genotypes. Improvement in cancellous bone architecture was seen with immediate DHT replacement that was enhanced in AR transgenic lines compared to WT. In contrast, there were only modest changes in all genotypes using the delayed treatment paradigm. With ORX in both paradigms, trabecular number was decreased while spacing increased. Thus, androgen therapy is effective for the prevention of endosteal and cancellous osteopenia primarily through its anti-resorptive properties, but shows little anabolic action as a therapeutic strategy to restore bone. Given the similarity in response to androgen treatment in both AR transgenic lines, overlapping expression profiles suggest that the target cells mediating androgen action in vivo are mature osteoblast/osteocytes. Combined, these results demonstrate that in the adult mouse, androgen treatment can reduce bone resorption but has little overall anabolic activity.
Keywords: osteoblast, osteocyte, testosterone, androgen receptor, resorption, anabolic
Introduction
Androgens have sometimes been characterized as anabolic agents that can increase bone mass by stimulation of bone formation [1–3], and may represent an important therapeutic class with the potential to rebuild lost bone. Bone loss is associated with a hypogonadal state in both women and men [4], and leads to the development of bone fragility and osteoporosis. Osteoporosis is generally characterized by a relative increase in bone resorption (mediated by osteoclasts) compared to bone formation (mediated by osteoblasts). Repletion of androgen-deficient males or androgen supplementation of osteoporotic females has been shown to reduce bone loss [5]. These observations have been reproduced in genetic models of androgen receptor dysfunction or with androgen ablation [for review, see 6], indicating an important role for androgen signaling in skeletal homeostasis.
While there is a clear role for androgen signaling during development in males that is important for sexually-dimorphic skeletal growth [7], androgen action in adult bone is less well understood. For example, androgen supplementation has little positive effects on bone formation in non-hypogonadal adults [8]. Clinical trials that have evaluated the effectiveness of androgen therapy to improve bone mineral density have been disappointing, with only the most hypogonadal patients benefiting [9, 10]. Additional confusion regarding the specific effects of androgen in skeletal homeostasis results from the use of testosterone as monotherapy. Testosterone, the major circulating androgen, can also serve as the substrate for the production of estradiol via aromatase activity. As a consequence, some testosterone action may result from estrogen receptor-dependent activation after conversion to 17-ß estradiol. Experimental strategies that employ GnRH agonists followed by treatment with testosterone coupled with an aromatase inhibitor have been used to determine the extent to which specific androgen signaling in the hypogonadal adult is anti-resorptive (i.e., inhibits bone resorptive activity) or anabolic (i.e., enhances bone formation). Conflicting results have been reported using such an approach and thus the extent of anabolic vs. anti-resorptive responses remains open to debate [11–13]. These results underscore the lack of understanding of the specific role for androgen in bone biology, and the distinct contribution of androgen to the maintenance of a healthy skeleton in the adult remains unclear.
Androgen action is mediated by androgen receptor (AR) and AR expression (gene and protein) is highest during the mineralization stage in the osteocyte population, determined during differentiation using a well characterized primary culture model [14]. In order to evaluate the cell-autonomous direct effects of androgen signaling, we have constructed transgenic animal models with skeletally-targeted overexpression of AR to increase sensitivity to endogenous androgens. Importantly, enhanced androgen action occurs as a consequence of enhanced AR signaling only in those cells with elevated levels of AR, without changes in circulating steroid levels and without systemic androgen administration [15, 16]. In both transgenic families, AR overexpression is controlled using fragments of the type I collagen promoter, with both distinct and overlapping expression profiles. With overexpression driven from the 2.3kb fragment, AR2.3-tg mice demonstrate AR overexpression targeted to mature osteoblasts and osteocytes for enhanced responsiveness in the bone compartment with the highest endogenous AR concentrations [14]. Characterization of AR2.3-tg males shows AR overexpression in mature osteoblasts is associated with the development of a low turnover bone phenotype, with compromised bone quality and strength [16] and cell autonomous inhibition of osteoblast differentiation mediated by inhibition of BMP signaling on the endocortical surface [17]. With transgene expression driven from the 3.6 kb fragment, AR3.6-tg mice show overexpression throughout the osteoblast lineage, including stem cells, immature osteoblasts and mature mineralizing osteoblasts and osteocytes [17–19]. Similar to AR2.3-tg mice, male AR3.6-tg mice also demonstrate a low turnover bone phenotype with compromised bone quality and strength [15]. Since both transgenic lines have enhanced androgen responsiveness in mature osteoblasts and osteocytes via AR overexpression, a comparison between the two families suggests that the predominant action of androgen in the skeleton produces a low turnover phenotype mediated by AR expression in that shared compartment (i.e., mature osteoblasts/osteocytes). Notably, the novel aspects of the consequences of unique AR overexpression in stem cells and immature osteoblasts is a body composition phenotype with reduced fat and increased muscle mass [19, 20], not observed in AR2.3-tg mice.
The goal of these studies was to evaluate the anabolic vs. anti-resorptive effects of dihydrotestosterone (DHT), a nonaromatizable androgen in hypogonadal male adult mice, using littermate control WT mice and both AR transgenic families with enhanced androgen responsiveness in bone. To optimize detection of anabolic effects vs. anti-resorptive effects of androgen treatment in the adult, two experimental paradigms were employed. In the first model, DHT treatment was initiated immediately after ORX during a period of higher turnover [21]. In the second model, ORX animals were allowed to develop bone loss first before DHT treatment was initiated, in a lower turnover setting [21–23]. By comparing results observed between these paradigms in all genotypes, we sought to test the effectiveness of DHT replacement to either prevent osteopenia during high turnover or to restore bone after hypogonadal loss as an anabolic strategy and the specific role of AR in the skeleton.
Materials and Methods
Animals
The generation of AR-transgenic mice employing the 2.3 kb α11 collagen promoter fragment to drive expression has been described previously [16]. AR2.3-tg and AR3.6-tg mice were bred to WT B6D2F1 mice (Jackson Labs, Bar Harbor, ME). Mice were maintained under a 12 h light-dark cycle, had free access to tap water and were fed ad libitum a standard rodent chow containing 4.5% fat and 23% protein (LabDiet 5001, PMI Nutrition Int., St. Louis, MO). All animal studies were performed according to institutional, local, state, federal and NIH guidelines for the use of animals in research under an Institutional Animal Use and Care Committee (IACUC)-approved protocol.
Experimental protocols
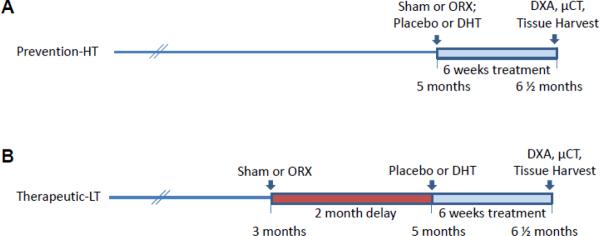
The effectiveness of androgen therapy to ameliorate hypogonadal loss was tested following ORX using male WT, AR2.3-tg and AR3.6-tg mice. Two experimental approaches were taken to emphasize potential anti-resorptive vs. anabolic actions of androgen replacement (see Fig. 1). In the first paradigm, protracted hormone ablation after ORX allowed the development of a hypogonadal phenotype that was then followed by androgen treatment. WT littermate control (B6D2F2) and AR transgenic mice were sham operated or orchidectomized at 3 months of age, and after a 2 month delay the effect of 6 weeks of treatment with nonaromatizable dihydrotestosterone (DHT) or placebo was determined. DHT treatment for 6 weeks is sufficient time to observe response to therapy, as major effects of androgen depletion and/or replacement on bone metabolism have been observed in rodents in just 3 weeks [24]. High turnover with rapid bone loss and low turnover with slow bone loss are established states of bone turnover that occur immediately or several months after gonadectomy in both humans and rodents [21–23, 25, 26]. Thus, after the two month delay, the high bone turnover typically observed after ORX has stabilized to a lower turnover state (LT). Treatments during LT will enhance the ability to detect anabolic signaling rather than anti-resorptive responses. In the LT hypogonadal state, hormone administration is considered a therapeutic intervention to restore lost bone. In the second paradigm, gonadecomy was performed at 5 months of age, followed immediately by DHT treatment again for 6 weeks. In this second approach, animals are in a high turnover (HT) state and treatment is characterized as a preventative intervention. Both LT and HT groups were evaluated at the end of the study at 6.5 months of age following 6 weeks of DHT treatment.
Figure 1.
Experimental protocol. Androgen therapy with the non-aromatizable androgen DHT was administered for the final 6 weeks of study in both paradigms. A. In prevention-HT, ORX was performed at 5 months of age and treatment initiated immediately. B. In the therapeutic-LT studies, ORX was performed at 3 months of age and treatment delayed for 2 months to allow a period of hypogonadal bone loss. Analysis in both groups was performed on 6½ month old mice.
Gonadectomy and steroid treatment
Anesthesia was induced with isoflurane (5% in air) and maintained with ~2% in air. For ORX, an incision was made through the skin at the tip of the scrotum on the left side and the testicular fat pad was localized and pulled through the incision using blunt forceps. A hemostat was placed across the testicular cord and the testes, epididymis and fat were removed with a Portable Cordless Cautery (Jorgensen Laboratories, Loveland, CO). The remaining pieces of tissue were replaced back in the scrotal sac and the incision closed with tissue adhesive (VetbondTM, 3M Animal Care Products, St. Paul, Minnesota, USA). The procedure was repeated for the right testes. Sham surgery involved making the same incision and then closing the wound. Upon completion, continuous release pellets (Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the interscapular region using a trochar. Pellets were either placebo (for both the sham and ORX control groups) or DHT prepared for continuous release over 60 days (12.5 mg or ~ 3 mg/kg/day). Confirmation of complete ORX was performed both by visual inspection at the end of the study and by bioassay using changes in weight of androgen-responsive tissues including seminal vesicle (SV) and levator ani bulbocavernosus muscle (LABC). This approach is considered more reliable in mice than determination of serum concentrations by RIA with problematic high variability [27, 28]. DHT levels were also characterized using the same androgen-responsive tissue weight as a bioassay, as previously described [20].
Tissue collection
All mice were weighed prior to sacrifice at 6½ months of age. SV and LABC muscle were harvested and wet weight determined. Individual femurs and the entire spine were dissected free of soft tissue from each animal and fixed in 70% ethanol for μCT analysis. Bones were stored at room temperature until analysis.
Bone mineral density and microstructure
The morphological and microstructural consequences of androgen treatment in both turnover paradigms were evaluated by dual energy X-ray absorptiometry (DXA) and quantitative μCT. Areal bone mineral density (BMD; mg/cm2), bone mineral content (BMC; mg) and bone area (cm2) were measured by whole body DXA using a mouse densitometer (PIXImus2, Lunar, Madison WI, USA). μCT was used for nondestructive three-dimensional evaluation of cortical and cancellous bone mass and architecture. Femora and 5th lumbar vertebrae were scanned using a Scanco μCT40 scanner (Scanco Medical AG, Basserdorf, Switzerland) at a voxel size of 12 × 12 × 12 μm. The threshold for analysis was determined empirically and set at 245 (scale 0 – 1000). Entire femora (cortical and cancellous bone) were evaluated followed by evaluation of cortical bone at the femoral midshaft diaphysis and cancellous bone in the distal femur metaphysis and epiphysis. For the femoral midshaft, 20 slices (240 μm) of bone were evaluated and total cross-sectional volume (cortical and marrow volume, μm3), cortical volume (μm3), marrow volume (μm3) and cortical thickness (μm) were measured. For the femoral metaphysis, 42 slices (504 μm) of secondary spongiosa were evaluated. Analysis of the lumbar vertebra included the entire region of secondary spongiosa between the cranial and caudal growth plates. Direct cancellous bone measurements in the distal femur and lumbar vertebra included: bone volume/tissue volume (BV/TV; %), trabecular number (Tb.N; mm−1), trabecular thickness (Tb.Th; μm) and trabecular separation (Tb.Sp; μm).
Histomorphometry methods
The histological methods used here have been described in detail [29]. In brief, 5th lumbar vertebrae were dehydrated in graded increases of ethanol and xylene and embedded undecalcified in methyl methacrylate. Sections (4-mm thick) were cut with a vertical bed microtome (Leica/Jung 2165), affixed to slides precoated with a 1% gelatin solution, and stained with toluidine blue (Sigma Chemical, St Louis, MO) for assessment of cartilage. The cancellous compartment was evaluated under visible light for presence of cartilage within cancellous bone and was scored blinded from 0 (absence of cartilage) to 3 (presence of extensive cartilage).
Statistical analysis
All data were analyzed using Prism5 software (GraphPad Software Inc., San Diego, CA). Significant differences among treatment groups were assessed by one-way ANOVA followed by post-hoc analysis with Newman-Keuls Multiple Comparison Test. Comparison between genotypes in sham animals was by unpaired two-tailed t-test. All data are expressed as mean ± standard error of the mean (SEM).
Results
The extent to which androgen therapy in the hypogonadal adult is anti-resorptive (i.e., inhibits bone resorptive activity) or anabolic (i.e enhances bone formation) remains open to debate [11, 12]. In order to evaluate the effectiveness of treatment with a nonaromatizable androgen (DHT), two treatment paradigms were developed that were optimized for the observation of either anabolic or anti-resorptive responses after orchiectomy. As shown in Fig. 1, the first treatment was considered as primarily prevention of bone loss in the high turnover setting that is observed immediately after gonadectomy (prevention-HT). The second paradigm is considered a therapeutic intervention, aimed at replacing bone lost after a sustained period of hypogonadal loss when turnover has stabilized (therapeutic-LT). Both treatment paradigms employed WT mice or transgenic mice with enhanced sensitivity to androgen resulting from AR overexpression targeted to the skeleton, using two independent AR-tg families with unique and overlapping expression profiles [15, 16].
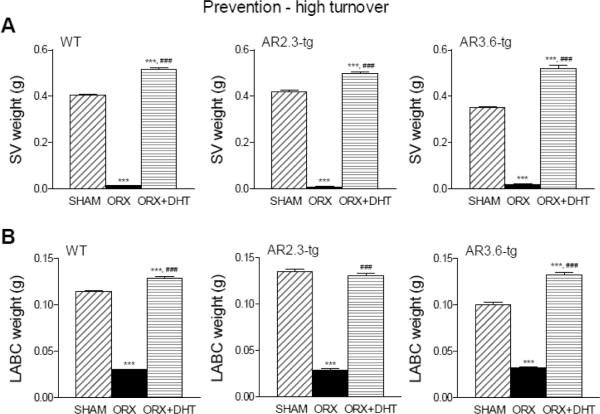
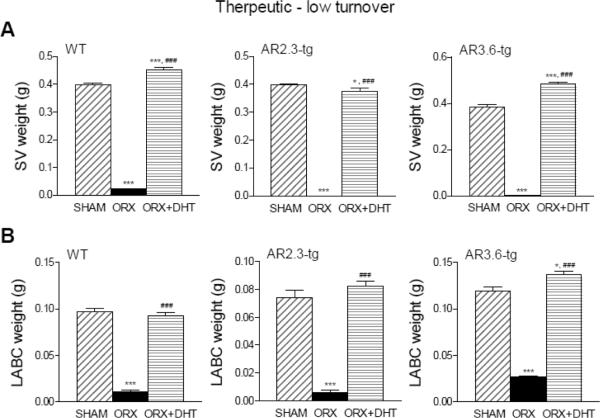
The effectiveness of gonadectomy was confirmed using androgen-responsive tissue weight as a sensitive bioassay for serum androgen concentrations in both paradigms. As shown in Fig. 2, WT, AR2.3-tg and AR3.6-tg mice employed in the prevention-HT paradigm showed significant losses in SV wet weight in the upper panels (reduced by >95% in all genotypes; all groups p < 0.001) and LABC wet weight in the lower panels (reduced >75% in all genotypes; all groups p < 0.001) after ORX. Similarly, in the therapeutic-LT model as shown in Fig. 3, all genotypes showed significant losses in SV wet weight in the upper panels (reduced by >95% in all genotypes; all groups p < 0.001) and LABC wet weight after ORX in the lower panels (reduced >75% in all genotypes; all groups p < 0.001). In addition, bioassay was used to evaluate circulating steroid concentrations that influence organ weight after androgen treatment. In WT AR2.3-tg and AR3.6-tg mice in both HT and LT paradigms, treatment with DHT constant release pellets restored lost weight in SV and LABC back to or above sham control levels, consistent with adequate androgen replacement (Figs. 2 and 3). Combined, these results confirm the effectiveness of both the gonadectomy surgery and androgen replacement.
Figure 2.
Bioassay in the prevention-HT paradigm in WT, AR2.3-tg and AR3.6-tg male mice using androgen-responsive tissue weight after ORX and with DHT treatment for 6 weeks. A. SV weight during HT intervention as prevention treatment. (n = 10–32). B. LABC muscle weight during HT. (n = 10–26). All data expressed as mean ± SEM. Significance was assessed by one-way ANOVA with Newman-Keuls Multiple Comparison post-hoc test or two-sided student's t-test. *** p < 0.001 vs. sham controls; ### p < 0.001 vs. ORX.
Figure 3.
Bioassay in LT mice using androgen-responsive tissue weight after ORX and with DHT treatment. A. SV weight during LT intervention as a therapeutic treatment for established bone loss. (n = 5–26). B. LABC muscle weight during HT. (n = 3–12). All data expressed as mean ± SEM. Significance was assessed by one-way ANOVA with Newman-Keuls Multiple Comparison post-hoc test or two-sided student's t-test. *** p < 0.001 vs. sham controls; ### p < 0.001 vs. ORX.
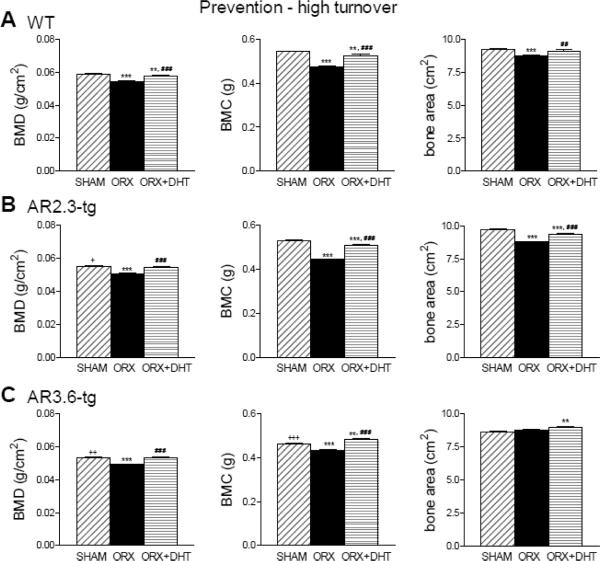
We first evaluated the effectiveness of androgen treatment to prevent rapid bone loss observed after ORX, using a nonaromatizable androgen (DHT). In the prevention-HT paradigm, gonadectomy was performed in 5 month old mice and DHT treatment was initiated immediately for 6 weeks. Changes in bone mineral were characterized by DXA analysis (Fig. 4). In WT male mice (upper panels), 6 weeks in a hypogonadal state after ORX results in a significant decrease in BMD (p < 0.001), BMC (p < 0.001) and bone area (p < 0.001). DHT treatment significantly increased BMD (p < 0.001), BMC (p < 0.001) and bone area (p < 0.01) all compared to ORX. DHT treatment did not completely prevent the loss of BMD or BMC as DHT treated WT males were still significantly lower than sham controls (both p < 0.01). Androgen treatment completely restored bone area, which was not significantly different between controls and ORX + DHT mice. Both AR2.3-tg and AR3.6-tg mice (middle and lower panels) also lost bone mineral following gonadectomy as BMD and BMC were all significantly reduced after ORX (p < 0.001 for all measures compared to sham controls). Bone area was also significantly reduced in AR2.3-tg mice (p < 0.001). DHT treatment ameliorated these changes (all p < 0.001 vs. ORX). In fact, DHT completely prevented the reduction in BMD with ORX in both AR2.3-tg and AR3.6-tg mice compared to controls. Thus, male mice of both genotypes lost significant amounts of bone mineral after ORX, and DHT was effective at ameliorating losses. Notably in both transgenic lines, DHT treatment completely prevented lost BMD during high turnover.
Figure 4.
Improvements in bone mineral with DHT treatment in ORX mice during HT intervention assessed by DXA analysis. WT male high turnover bone mineral measures assessed by DXA (Upper panel,). A. Changes in WT mice in whole body BMD, BMC and total bone area. (n = 9–20). B. Assessment in AR2.3-tg mice of whole body BMD, BMC and total bone area. (n = 14–16). C. Assessment in AR3.6-tg mice of whole body BMD, BMC and total bone area. (n = 9–12). All data expressed as mean ± SEM. ** p < 0.01, *** p < 0.001 vs. sham controls; ## p < 0.01, ### p < 0.001 vs. ORX; + p < 0.05, ++ p < 0.01, +++ p < 0.001 vs. WT sham.
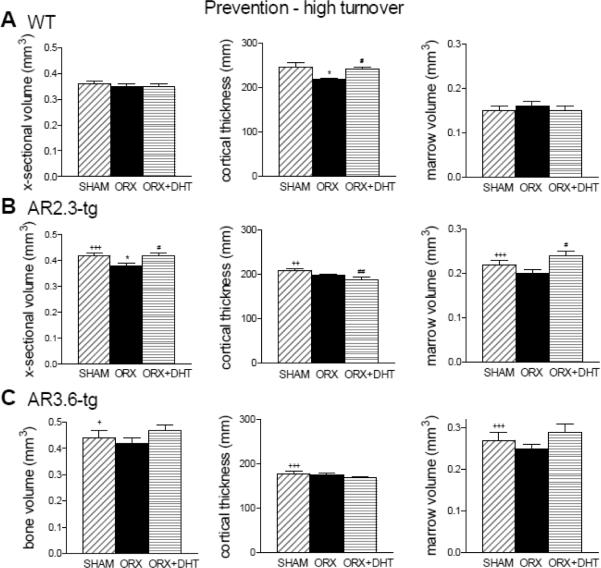
Cortical bone microstructure was then characterized by μCT at the femoral mid-diaphysis in the high turnover paradigm. As shown (Fig. 5), there were reductions in cortical thickness with ORX in WT mice (upper panels). Changes in cross-sectional volume or marrow volume did not reach significance. DHT treatment prevented the decrease in cortical thickness in WT male mice following ORX. AR2.3-tg mice (middle panel) show a more robust response to DHT replacement in the ORX males, with significantly increased both cross-sectional and marrow volume (both p < 0.05 vs. ORX). Notably, with AR overexpression, DHT treatment did not increase cortical thickness compared to ORX as was noted in WT mice. This result in the adult transgenic male at 6.5 months of age is consistent with decreased endocortical bone formation observed during development [15, 16]. Differences in cortical thickness, cross-sectional volume, or marrow volume did not reach significance with treatment in AR3.6-tg mice (lower panel).
Figure 5.
Improvements in mid-diaphyseal cortical bone volume with DHT treatment during HT intervention as assessed by μCT analysis. A. Changes in WT mice in femoral bone volume, cortical thickness and marrow volume. (n = 8–10). B. Assessment of mid-diaphyseal bone in AR2.3-tg mice by μCT analysis. (n = 7–14). C. Analysis of mid-diaphyseal bone in AR3.6-tg mice. (n = 5–13). All data expressed as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. sham controls; # p < 0.05, ## p < 0.01 vs. ORX; + p < 0.05, ++ p < 0.01, +++ p < 0.001 vs. WT sham.
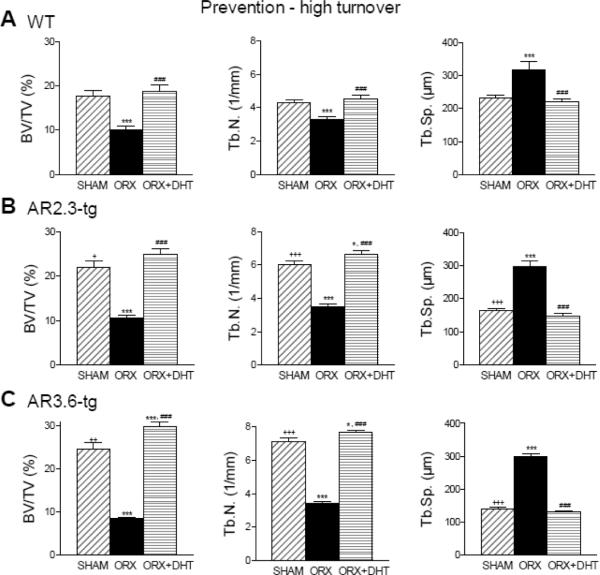
Cancellous bone microarchitecture during high turnover was then characterized by μCT at three sites; vertebrae, femoral metaphysis and femoral epiphysis. Similar responses were observed at all three sites; we show here responses in lumbar vertebrae (Fig. 6). In cancellous bone, ORX dramatically reduced BV/TV (p < 0.001) which was completely prevented with DHT treatment (p < 0.001 vs. ORX in all genotypes). In AR3.6-tg mice, enhanced sensitivity to androgen was associated with a significant increase in BV/TV compared to WT (p < 0.001). Tb.N. was reduced and conversely Tb.Sp. was increased with ORX compared to sham (p < 0.001 for all genotypes). DHT treatment completely prevented loss of cancellous bone through an increase in Tb.N., a hallmark of anti-resorptive treatment. Both AR2.3-tg and AR3.6-tg mice were more responsive than WT mice since androgen treatment significantly increased Tb.N compared to control animals (p < 0.05) but did not in WT mice..
Figure 6.
Characterization of cancellous bone microarchitecture by μCT analysis during HT treatment. Analysis shown was performed in lumbar vertebrae. A. Prevention of cancellous bone loss in WT mice in BV/TV, Tb.N. and Tb.Sp. (n = 9–13). B. Similar prevention of cancellous bone loss in AR2.3-tg mice by immediate DHT treatment (n = 11–15). C. Immediate DHT treatment for prevention of cancellous bone loss in AR3.6-tg mice (n = 12–15). All data expressed as mean ± SEM. * p < 0.05, *** p < 0.001 vs. sham controls; ### p < 0.001 vs. ORX; + p < 0.05, ++ p < 0.01, +++ p < 0.001 vs. WT sham.
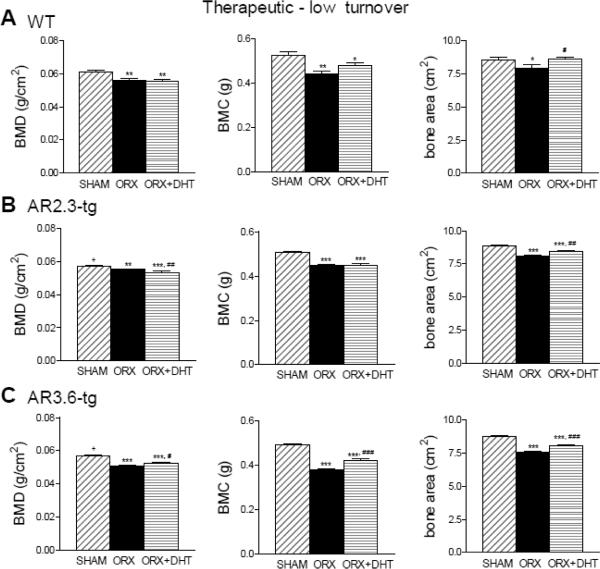
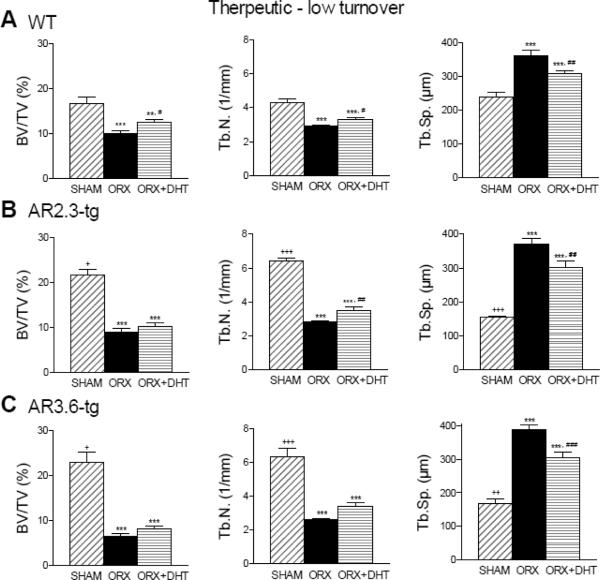
In the next set of studies, we evaluated the effectiveness of androgen as an anabolic agent in the therapeutic paradigm, testing the ability of DHT treatment to restore bone after a defined period of hypogonadal bone loss. Adult mice were gonadectomized at 3 months of age and left for two months before DHT treatment, again for 6 weeks. Bone mineral was assessed by DXA (Fig. 7). In this model of established bone loss in a low turnover setting, a sustained period of hypogonadal bone loss after ORX resulted in significantly reduced BMD, BMC and bone area in all genotypes compared with control. DHT treatment provided no benefit to normalize BMD or BMC in WT, but in AR3.6 mice treatment did modestly improve BMD, BMC and bone area compared to ORX. Notably, in AR2.3-tg mice, DHT treatment further reduced BMD compared to both ORX (p < 0.01) and sham controls (p < 0.001), and did not increase BMC. Thus, in striking contrast to results observed in high turnover setting (see Fig. 4), DHT treatment provided only modest overall benefit.
Figure 7.
Androgen treatment does not restore bone mineral in ORX mice during LT intervention as assessed by DXA analysis. A. Changes in WT mice in whole body BMD, BMC and total bone area (n = 10–13). B. Assessment in AR2.3-tg mice (n = 10). C. Analysis in AR3.6-tg mice (n = 7–10). All data expressed as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. sham control; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. ORX; + p < 0.05 vs. WT sham.
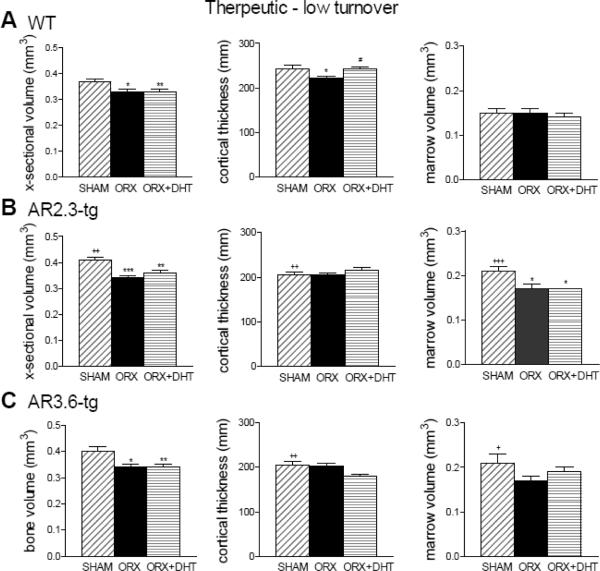
Cortical bone microstructure at the femoral mid-diaphysis was characterized by μCT analysis, here to evaluate potential anabolic responses (Fig. 8). In this setting, ORX reduced cross-sectional volume in WT mice (p < 0.01 vs. sham) and both transgenic mice (p < 0.05), and also reduced cortical thickness in WT mice (p < 0.05 vs. sham). DHT treatment significantly increased cortical thickness in WT mice compared to ORX alone (p < 0.05), but in both transgenic lines DHT treatment did not improve cortical thickness. A reduction in marrow volume was observed in AR2.3-tg mice after ORX (p < 0.05) that was not influenced by DHT treatment. DHT thus demonstrated little anabolic activity on cortical bone architecture in the low turnover setting.
Figure 8.
Minimal improvement in mid-diaphyseal cortical bone volume with DHT treatment during LT intervention as assessed by μCT analysis. A. Changes in WT mice in femoral bone volume, cortical thickness and marrow volume (n = 10–11). B. Assessment of mid-diaphyseal bone in AR2.3-tg mice (n = 7–11). C. Analysis of mid-diaphyseal bone by μCT in AR3.6-tg mice (n = 8–11). All data expressed as mean ± SEM. * p < 0.05, ** p < 0.01 vs. sham control; # p < 0.05; + p < 0.05, ++ p < 0.01 vs. WT sham.
Cancellous analysis by μCT in the low turnover setting (Fig. 9) showed that ORX resulted in significant losses in BV/TV and Tb.N. (p < 0.001 for both measures for all genotypes compared to sham), and increased Tb.Sp. (p < 0.001 for all genotypes compared to sham). DHT treatment had no significant effect to restore lost BV/TV in either transgenic line but there was a modest increase in WT mice (p < 0.05 vs. ORX). There were also modest improvements in all genotypes in changes in Tb.N. and Tb.Sp. with DHT treatment. This result contrasts with the robust response to DHT to prevent cancellous loss in the high turnover setting in WT and even greater response in AR transgenic mice (see Fig. 6).
Figure 9.
Characterization of cancellous bone architecture by μCT analysis during LT treatment. Analysis shown was performed in lumbar vertebrae. A. Loss of cancellous bone in WT mice in BV/TV, Tb.N. and Tb.Sp. (n = 12–19). B. AR2.3-tg mice show the same cancellous bone loss even after DHT treatment (n = 12). C. Cancellous bone loss in AR3.6-tg mice (n = 9–12). All data expressed as mean ± SEM. ** p < 0.01, *** p < 0.001 vs. sham controls; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. ORX; + p < 0.05, ++ p < 0.01, +++ p < 0.001 vs. WT sham.
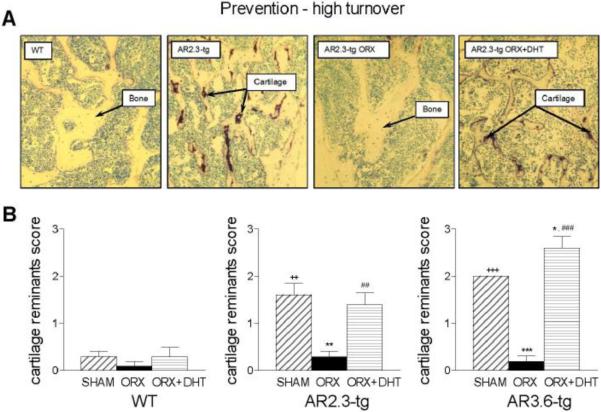
Given the robust response to prevent bone loss in the prevention-HT paradigm but lack of response in the therapeutic-LT animals, histomorphometric analysis was performed in the higher turnover group. Histomophometry was used to characterize retention of cartilage fragments, a hallmark of impaired bone resorption, to provide a direct assessment of bone resorption in vivo. Analysis was performed as previously described [29] within cancellous bone in lumbar vertebrae, to evaluate the effects of AR overexpression, ORX, and DHT treatment. Representative images are shown in Fig. 10A. Cartilage was rare in WT mice and when observed, was predominantly confined to areas immediately adjacent to the growth plate. Neither ORX nor DHT treatment significantly altered the amount of cartilage in WT mice (quantitative analysis Fig. 10B). In contrast, higher levels of cartilage remnants were present in both AR transgenic lines. In AR2.3-tg and AR3.6-tg male mice, cartilage retention was significantly higher compared to WT sham control mice (p < 0.01 and p < 0.001, respectively). As expected, ORX resulted in a significant reduction of cartilage remnants in both AR2.3-tg and AR3.6-tg lines (p < 0.01 and p < 0.001, respectively). Notably, immediate administration of DHT inhibited resorption and preserved the remnants in both lines (p < 0.01 and p < 0.001, respectively vs. ORX).
Figure 10.
Characterization of WT and AR-tg families for retention of cartilage tissue in cancellous bone by histomorphometric analyses as a bioassay for resorptive activity in bone tissue. Analysis was performed in lumbar vertebra. A. Representative photos of WT sham and AR2.3-tg sham, ORX and DHT treated. B. Quantitative analysis of cartilage remnants in WT, AR2.3-tg and AR3.6-tg mice (n = 5). Retention of cartilage was scored blinded from 0 (absence of cartilage) to 3 (presence of extensive cartilage). All data expressed as mean ± SEM. ** p < 0.01, *** p < 0.001 vs. sham controls; ## p < 0.01, ### p < 0.001 vs. ORX; ++ p < 0.01, +++ p < 0.001 vs. WT sham.
Discussion
The consequences of androgen replacement in low and high turnover states were evaluated in WT mice and contrasted with AR2.3-tg and AR3.6-tg mice, models of enhanced androgen responsiveness with skeletal targeting. A comparison between results observed in the two paradigms indicates that androgen therapy is effective at preventing osteopenia only when administered immediately after gonadectomy. In the prevention-HT model, ORX significantly reduced BMD and BMC in all genotypes compared to sham and DHT was more effective at prevention of loss in both families of AR transgenic mice. Cancellous microstructure analysis by μCT showed ORX resulted in significant losses in BV/TV and Tb.N. and immediate DHT replacement prevented trabecular loss in all genotypes but was significantly more effective to increase cancellous bone in AR transgenic mice compared with control. Retention of cartilage fragments after ORX was also observed in both AR transgenic lines with immediate DHT treatment as a direct demonstration of anti-resorptive activity. In contrast, analysis of the effects of gonadectomy and DHT treatment in the therapeutic model showed that although ORX significantly reduced BMD and BMC in all genotypes compared to sham, delayed DHT treatment provided little benefit in any genotype. Microstructure analysis in cancellous bone showed ORX resulted in significant losses in BV/TV in all genotypes and there was only modest improvement with DHT treatment. Thus, the consequence of androgen treatment on skeletal homeostasis via androgen receptor signaling in the adult is primarily anti-resorptive in vivo. Further, given the consistency of the response between the two transgenic lines and overlap in overexpression profiles, these results also indicate that the target cells mediating this response are mature osteoblast/osteocytes.
Here we report a comparison between two treatment paradigms to evaluate bone loss after gonadectomy, optimized to observe potential anabolic vs. anti-resorptive responses of androgen therapy. We performed ORX in sexually-mature mice at 3 or 5 months of age, as the rapid pre-pubertal and pubertal growth phase in mice has slowed by 2 months of age [30]. In the prevention-HT arm, bone turnover is high after gonadectomy[21]. High turnover is also observed in humans immediately after menopause or gonadectomy [25]. In both humans and rodents, turnover rates decline with time [21–23, 26]. In the therapeutic-LT arm, the response to therapy is evaluated after turnover has declined. In this setting, significant bone loss has occurred before treatment is initiated. DHT was administered with constant release pellets over 6 weeks, which has previously been shown to be sufficient time for treatment after gonadectomy to observe changes in bone histomorphometry [31] and in cancellous bone mass [32]. The dose of DHT (~3 mg/kg/day) was selected based on previous work [33], and resulted in seminal vesicle and LABC weights that returned to or exceeded sham control levels after the hypogonadal period. Our results demonstrate the effectiveness of androgen administration to prevent bone loss but there were negligible effects consistent with anabolic activity.
The effectiveness of androgen treatment to increase bone mass and mineral has been evaluated in a variety of clinical trials [8, 10]. In general, these reports indicate that androgen treatment does little to increase bone mass. Given the ability of androgen to suppress bone resorption, clinical trials evaluating androgen therapy may especially benefit from selective targeting of a higher turnover phenotype. Only bone derived from neural crest origins, such as frontal bone of the calvaria or the periosteum, demonstrate an anabolic increase in bone formation in response to androgen [15, 18]. Turner and colleagues clearly demonstrated that androgens stimulate periosteal bone formation in growing rodents while inhibiting cancellous bone formation [34]. Also, androgens suppress bone formation in endocortical bone [15, 16]; this inhibition is more robust in AR2.3-tg mice, as cortical thickness did not increase and in fact marrow volume was elevated in response to DHT treatment in the high turnover paradigm.
The osteocyte is the most abundant cell type present in bone tissue and are the only cells embedded in the calcified bone matrix. It has been suggested an important role of the osteocyte is to regulate bone resorption [35]. The osteocyte is a likely target of androgen action in the skeleton since the highest concentrations of receptor are present in mineralizing cultures [14]. Data presented here suggests that androgen can regulate bone resorption and that when signaling is enhanced in the mature osteoblast/osteocyte population in both AR transgenic lines, more robust inhibition of cancellous loss is observed, consistent with the notion that a major function for androgen in the skeleton is to control turnover [36]. These findings are in agreement with Wakely et al [24] who demonstrated that ORX-induced bone loss is associated with increases in osteoblast and osteoclast perimeter. Furthermore, dose response studies revealed that physiological replacement of testosterone prevented bone loss and normalized bone turnover whereas high levels of testosterone or DHT led to further suppression of osteoclasts with minimal effects on bone mass. Combined, these results suggest that androgen action can inhibit bone resorption potentially through changes in osteocyte activity.
The effect of androgen is undoubtedly complex, but reports indicate that androgens can inhibit bone resorption via effects on the receptor activator of NF-kB ligand (RANKL)/RANK/OPG system [see 37]. We have previously shown significant reductions in RANKL and TRAP gene expression in diaphyseal bone samples from AR2.3-tg males [16]. The retention of cartilage cores within the cancellous bone compartment of both AR overexpressing lines dramatically illustrates the AR-mediated inhibition of bone resorption in vivo. Other AR genetic models strongly implicate androgens in control of bone resorption. For example, the bone phenotype that develops in an AR null (ARKO) mouse model, with a reduction in cancellous bone volume, is described as a high turnover state compared with WT littermates. In addition, conditional ablation of AR in mature osteoblasts also reduced bone mass, and a reduction in Tb.N. suggests that the bone loss was due to increased resorption [38]. In both AR transgenic lines, the reduction in Tb.N. observed with ORX was not only completely prevented in the high turnover setting, DHT treatment actually increased Tb.N. above the level observed in sham control mice. Although the above studies imply that the main action of androgens on the mature skeleton is to inhibit bone resorption, the failure of DHT to restore bone in ORX mice with established bone loss shown here provides compelling additional evidence for this androgen action. There were only modest improvements in any genotype in the low turnover setting. Combined, the data shown here support the importance of androgen signaling through the AR to reduce bone turnover, likely through osteocyte-mediated inhibition of osteoclastogenesis or activity.
In summary, the effectiveness of DHT treatment after ORX was evaluated in two settings where bone turnover state varied; the prevention-HT or therapeutic-LT paradigms. In cortical bone, androgen was effective at reducing bone loss in the prevention-HT study but provided little benefit when DHT treatment was delayed. AR transgenic males generally did not improve as much as WT mice in this compartment. In metabolically active cancellous bone, the effect of ORX and immediate intervention was more robust. With delayed treatment, there was no net effect of DHT to restore BV/TV, but when administered at the time of ORX, DHT completely prevented trabecular loss in all genotypes. Notably, DHT treatment in both AR transgenic lines was generally more effective than in WT, consistent with the suggestion that androgen signaling in the mature osteoblast/osteocyte can regulate osteoclastic activity to modulate bone resorption. Thus, androgen therapy is effective for the prevention of bone loss primarily through its anti-resorptive properties, but shows little anabolic action as a therapeutic strategy to restore lost bone as only small improvements are seen. The response was compartment-specific, with significant elevations in cancellous bone with androgen treatment but DHT was less effective in cortical bone, particularly in low turnover. Increased sensitivity in mature osteoblasts/osteocytes does not increase anabolic activity and instead likely enhances anti-resorptive responses observed in the high turnover model. Combined, these results indicate that in the adult, androgens acting on androgen receptors can reduce bone resorption but have little overall anabolic activity.
Highlights
Comparisons between wild-type mice and two distinct androgen receptor transgenic lines reveal cellular targets of androgen action
Two treatment paradigms employed to detect anti-resorptive vs. anabolic androgen activity with hypogonadal bone loss
Androgen treatment provided little anabolic activity in hypogonadal mice
Results show mature osteoblasts/osteocytes are a major target in vivo to mediate anti-resorptive effects of androgen
Acknowledgments
We thank Dr. Robert Klein (OHSU) for the use of the Piximus II work station for DXA analysis. We also thank Anthony A. Semirale for assistance with surgeries and DXA analysis. This publication was made possible by grants from the National Institute of Diabetes, Digestive & Kidney Disease R01 DK067541 (KMW) and the United States Army Research Acquisition Activity Award No. W81XWH-05-1-0086 (KMW). KMW is a Research Career Scientist in the Department of Veterans Affairs and received support from the Veterans Affairs Merit Review Program. All work was performed in facilities provided by the Department of Veterans Affairs.
Abbreviations
- AR2.3-tg
AR2.3-transgenic
- AR3.6-tg
AR3.6-transgenic
- BMC
bone mineral content
- BMD
bone mineral density
- BV/TV
bone volume/tissue volume
- DXA
dual-energy x-ray absorptiometry
- DHT
5α-dihydrotestosterone
- LABC
combined levator ani bulbocavernosus muscle
- ORX
orchidectomy
- SV
seminal vesicle
- Tb.N.
trabecular number
- Tb.Sp.
trabecular spacing
- μCT
microComputed tomography
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kenny A, Raisz L. Mechanisms of bone remodeling: implications for clinical practice. J Reprod Med. 2002;47:63–70. [PubMed] [Google Scholar]
- [2].Liegibel U, Sommer U, Tomakidi P, Hilscher U, Van Den Heuvel L, Pirzer R, et al. Concerted action of androgens and mechanical strain shifts bone metabolism from high turnover into an osteoanabolic mode. J Exp Med. 2002;196:1387–1392. doi: 10.1084/jem.20021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Orwoll E. Androgens as anabolic agents for bone. Trends Endocrinol Metab. 1996;7:77–84. doi: 10.1016/1043-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- [4].Greenspan S, Oppenheim D, Klibanski A. Importance of gonadal steroids to bone mass in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1989;110:526–531. doi: 10.7326/0003-4819-110-7-526. [DOI] [PubMed] [Google Scholar]
- [5].Raisz LG, Wiita B, Artis A, Bowen A, Schwartz S, Trahiotis M, et al. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37–43. doi: 10.1210/jcem.81.1.8550780. [DOI] [PubMed] [Google Scholar]
- [6].Wiren KM. Androgens and bone growth: it's location, location, location. Curr Opin Pharmacol. 2005;5:626–632. doi: 10.1016/j.coph.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [7].Vignozzi L, Morelli A, Filippi S, Maggi M, Forti G. Male pubertal development: role of androgen therapy on bone mass and body composition. J Endocrinol Invest. 2010;33:27–32. [PubMed] [Google Scholar]
- [8].Wang C, Cunningham GR, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- [9].Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20:1785–1791. doi: 10.1359/JBMR.050606. [DOI] [PubMed] [Google Scholar]
- [10].Kenny A, Kleppinger A, Annis K, Rathier M, Browner B, Judge J, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sanyal A, Hoey K, Modder U, Lamsam J, McCready L, Peterson J, et al. Regulation of bone turnover by sex steroids in men. J Bone Miner Res. 2008;23:705–714. doi: 10.1359/JBMR.071212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leder B, LeBlanc K, Schoenfeld D, Eastell R, Finkelstein J. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- [13].Falahati-Nini A, Riggs B, Atkinson E, O'Fallon W, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wiren KM, Chapman Evans A, Zhang XW. Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol. 2002;175:683–94. doi: 10.1677/joe.0.1750683. [DOI] [PubMed] [Google Scholar]
- [15].Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, et al. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology. 2004;145:3507–22. doi: 10.1210/en.2003-1016. [DOI] [PubMed] [Google Scholar]
- [16].Wiren KM, Semirale AA, Zhang XW, Woo A, Tommasini SM, Price C, et al. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis: a model for compartment-specific androgen action in the skeleton. Bone. 2008;43:440–51. doi: 10.1016/j.bone.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wiren KM, Semirale AA, Hashimoto JG, Zhang XW. Signaling pathways implicated in androgen regulation of endocortical bone. Bone. 2010;46:710–723. doi: 10.1016/j.bone.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wiren KM, Hashimoto JG, Semirale AA, Zhang XW. Bone vs. fat: Embryonic origin of progenitors determines response to androgen in adipocytes and osteoblasts. Bone. 2011;49:662–672. doi: 10.1016/j.bone.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Semirale AA, Zhang XW, Wiren KM. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem. 2011;112:1773–1786. doi: 10.1002/jcb.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wiren KM, Hashimoto JG, Zhang XW. Stem cell activation in adults can reverse detrimental changes in body composition to reduce fat and increase lean mass in bothsexes. J Cell Biochem. 2011;112:3638–3647. doi: 10.1002/jcb.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vanderschueren D, Van Herck E, Suiker A, Visser W, Schot L, Bouillon R. Bone and mineral metabolism in aged male rats: Short and long term effects of androgen deficiency. Endocrinology. 1992;130:2906–2916. doi: 10.1210/endo.130.5.1572302. [DOI] [PubMed] [Google Scholar]
- [22].Vanderschueren D, Jans I, van Herck E, Moermans K, Verhaeghe J, Bouillon R. Time-related increase of biochemical markers of bone turnover in androgen-deficient male rats. J Bone Miner Res. 1994;26:123–131. doi: 10.1016/s0169-6009(08)80057-8. [DOI] [PubMed] [Google Scholar]
- [23].Verhas M, Schoutens A, L'hermite-Baleriaux M, Dourov N, Verschaeren A, Mone M, et al. The effect of orchidectomy on bone metabolism in aging rats. Calcif Tissue Int. 1986;39:74–77. doi: 10.1007/BF02553294. [DOI] [PubMed] [Google Scholar]
- [24].Wakley G, Schutte H, Hannon K, Turner R. Androgen treatment prevents loss of cancellous bone in the orchidectomized rat. J Bone Miner Res. 1991;6:325–330. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- [25].Simon J, Wehren L, Ascott-Evans B, Omizo M, Silfen S, Lombardi A. Skeletal consequences of hormone therapy discontinuance: a systematic review. Obstet Gynecol Surv. 2006;61:115–124. doi: 10.1097/01.ogx.0000189152.95070.f8. [DOI] [PubMed] [Google Scholar]
- [26].Luisetto G, Zangari M, Tizian L, Nardi A, Ramazzina E, Adami S, et al. Influence of aging and menopause in determining vertebral and distal forearm bone loss in adult healthy women. Bone Miner. 1993;22:9–25. doi: 10.1016/s0169-6009(08)80077-3. [DOI] [PubMed] [Google Scholar]
- [27].Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- [28].Sacks S. Are routine testosterone assays good enough? Clin Biochem Rev. 2005;26:43–45. [PMC free article] [PubMed] [Google Scholar]
- [29].Iwaniec U, Wronski T, Turner R. Histological analysis of bone. Methods Mol Biol. 2008;447:325–341. doi: 10.1007/978-1-59745-242-7_21. [DOI] [PubMed] [Google Scholar]
- [30].Richman C, Kutilek S, Miyakoshi N, Srivastava A, Beamer W, Donahue L, et al. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res. 2001;16:386–397. doi: 10.1359/jbmr.2001.16.2.386. [DOI] [PubMed] [Google Scholar]
- [31].Gunness M, Orwoll E. Early induction of alterations in cancellous and cortical bone histology after orchiectomy in mature rats. J Bone Miner Res. 1995;10:1735–1744. doi: 10.1002/jbmr.5650101117. [DOI] [PubMed] [Google Scholar]
- [32].Tivesten A, Moverare-Skrtic S, Chagin A, Venken K, Salmon P, Vanderschueren D, et al. Additive protective effects of estrogen and androgen treatment on trabecular bone in ovariectomized rats. J Bone Miner Res. 2004;19:1833–1839. doi: 10.1359/JBMR.040819. [DOI] [PubMed] [Google Scholar]
- [33].Ma J, Graves J, Bradbury J, Zhao Y, Swope D, King L, et al. Regulation of Mouse Renal CYP2J5 Expression by Sex Hormones. Mol Pharmacol. 2004;65:730–743. doi: 10.1124/mol.65.3.730. [DOI] [PubMed] [Google Scholar]
- [34].Turner R, Hannon K, Demers L, Buchanan J, Bell N. Differential effects of gonadal function on bone histomorphometry in male and female rats. J Bone Miner Res. 1989;4:557–563. doi: 10.1002/jbmr.5650040415. [DOI] [PubMed] [Google Scholar]
- [35].Henriksen K, Neutzsky-Wulff A, Bonewald L, Karsdal M. Local communication on and within bone controls bone remodeling. Bone. 2009;44:1026–1033. doi: 10.1016/j.bone.2009.03.671. [DOI] [PubMed] [Google Scholar]
- [36].Frenkel B, Hong A, Baniwal S, Coetzee G, Ohlsson C, Khalid O, et al. Regulation of adult bone turnover by sex steroids. J Cell Physiol. 2010;224:305–310. doi: 10.1002/jcp.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- [38].Notini A, McManus J, Moore A, Bouxsein M, Jimenez M, Chiu W, et al. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22:347–356. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]