Abstract
Most biogerontologists agree that oxygen (and nitrogen) free radicals play a major role in the process of aging. The evidence strongly suggests that the electron transport chain, located in the inner mitochondrial membrane, is the major source of reactive oxygen species in animal cells. It has been reported that there exists an inverse correlation between the rate of superoxide/hydrogen peroxide production by mitochondria and the maximum longevity of mammalian species. However, no correlation or most frequently an inverse correlation exists between the amount of antioxidant enzymes and maximum longevity. Although overexpression of the antioxidant enzymes SOD1 and CAT (as well as SOD1 alone) have been successful at extending maximum lifespan in Drosophila, this has not been the case in mice. Several labs have overexpressed SOD1 and failed to see a positive effect on longevity. An explanation for this failure is that there is some level of superoxide damage that is not preventable by SOD, such as that initiated by the hydroperoxyl radical inside the lipid bilayer, and that accumulation of this damage is responsible for aging. I therefore suggest an alternative approach to testing the free radical theory of aging in mammals. Instead of trying to increase the amount of antioxidant enzymes, I suggest using molecular biology/transgenics to decrease the rate of superoxide production, which in the context of the free radical theory of aging would be expected to increase longevity. This paper aims to summarize what is known about the nature and mechanisms of superoxide production and what genes are involved in controlling the rate of superoxide production.
Full Text
The Full Text of this article is available as a PDF (6.8 MB).
References
- 1.Beckman K.B., Ames B.N. Mitochondrial aging: open questions. Ann. N. Y. Acad. Sci. 1998;854:118–127. doi: 10.1111/j.1749-6632.1998.tb09897.x. [DOI] [PubMed] [Google Scholar]
- 2.Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 3.Beckman K.B., Ames B.N. Endogenous oxidative damage of mtDNA. Mutat. Res. 1999;424:51–58. doi: 10.1016/s0027-5107(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 4.Orr W.C., Sohal R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 5.Parkes T.L., Ella A.J., Dickinson D., Hilliker A.J., Phillips J.P., Boulianne G.L. Extension of Drosophila lifespan by overexpression of human SOD1 in motomeurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 6.Elia A.J., Parkes T.L., Kirby K., St. George-Hyslop P., Boulianne G.L., Phillips J.P., Hilliker A.J. Expression of human FALS SOD in motorneurons of Drosophila. Free Radic. Biol. Med. 1999;26:1332–1338. doi: 10.1016/S0891-5849(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 7.Reveillaud I., Niedzwiecki A., Bensch K.G., Fleming J.E. Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol. Cell. Biol. 1991;11:632–640. doi: 10.1128/mcb.11.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J., Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staveley B.E., Phillips J.P., Hilliker A.J. Phenotypic consequences of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990;33:867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- 10.Seto N.O., Hayashi S., Tener G.M. Overexpression of Cu-Zn superoxide dismutase in Drosophila does not affect life-span. Proc. Natl. Acad. Sci. U S A. 1990;87:4270–4274. doi: 10.1073/pnas.87.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang T.T., Carlson E.J., Gillespie A.M., Shi Y., Epstein C.J. Ubiquitous overexpression of Cu,Zn superoxide dismutase does not extend life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B5–9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- 12.Sohal R.S., Svensson I., Sohal B.H., Brunk U.T. Superoxide anion radical production in different animal species. Mech. Ageing Dev. 1989;49:129–135. doi: 10.1016/0047-6374(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 13.McFadden S.L., Ding D., Burkard R.F., Jiang H., Reaume A.G., Flood D.G., Salvi R.J. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J. Comp. Neurol. 1999;413:101–112. doi: 10.1002/(SICI)1096-9861(19991011)413:1<101::AID-CNE7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.McFadden S.L., Ding D., Reaume A.G., Flood D.G., Salvi R.J. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol. Aging. 1999;20:1–8. doi: 10.1016/S0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 15.Reaume A.G., Elliott J.L., Hoffman E.K., Kowall N.W., Ferrante R.J., Siwek D.F., Wilcox H.M., Flood D.G., Beal M.F., Brown R.H., Jr., Scott R.W., Snider W.D. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 16.Melov S., Coskun P., Patel M., Tuinstra R., Cottrell B., Jun A.S., Zastawny T.H., Dizdaroglu M., Goodman S.I., Huang T.T., Miziorko H., Epstein C.J., Wallace D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U S A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsan M.F., White J.E., Caska B., Epstein C.J., Lee C.Y. Susceptibility of heterozygous MnSOD gene-knockout mice to oxygen toxicity. Am. J. Respir. Cell. Mol. Biol. 1998;19:114–120. doi: 10.1165/ajrcmb.19.1.3066. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 19.Melov S., Schneider J.A., Day B.J., Hinerfeld D., Coskun P., Mirra S.S., Crapo J.D., Wallace D.C. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 20.Tolmasoff J.M., Ono T., Cutler R.G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc. Natl. Acad. Sci. U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Campo R., Lopez-Torres M., Rojas C., Cadenas S., Barja G. Longevity and antioxidant enzymes, non-enzymatic antioxidants and oxidative stress in the vertebrate lung: a comparative study. J. Comp. Physiol. [B] 1994;163:682–689. doi: 10.1007/BF00369520. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Campo R., Lopez-Torres M., Rojas C., Cadenas S., Barja G. A comparative study of free radicals in vertebrates—I. Antioxidant enzymes. Comp. Biochem. Physiol. [B] 1993;105:749–755. doi: 10.1016/0305-0491(93)90116-M. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Campo R., Lopez-Torres M., Cadenas S., Rojas C., Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J. Comp. Physiol. [B] 1998;168:149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Torres M., Perez-Campo R., Rojas C., Cadenas S., Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech. Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-U. [DOI] [PubMed] [Google Scholar]
- 25.Barja G., Cadenas S., Rojas C., Perez-Campo R., Lopez-Torres M. Low mitochondrial free radical production per unit O2 consumption can explain the simultaneous presence of high longevity and high aerobic metabolic rate in birds. Free Radic. Res. 1994;21:317–327. doi: 10.3109/10715769409056584. [DOI] [PubMed] [Google Scholar]
- 26.Sohal R.S., Svensson I., Brunk U.T. Hydrogen peroxide production by liver mitochondria in different species. Mech. Ageing Dev. 1990;53:209–215. doi: 10.1016/0047-6374(90)90039-I. [DOI] [PubMed] [Google Scholar]
- 27.Ku H.H., Brunk U.T., Sohal R.S. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic. Biol. Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-Q. [DOI] [PubMed] [Google Scholar]
- 28.Rubner M. Das Problem der Lebensdauer und seine Bezieung zu Wachstum und Ernehrung. Munich: Oldenburg; 1908. [Google Scholar]
- 29.Pearl R. The rate of living. London: University of London Press; 1928. [Google Scholar]
- 30.Ku H.H., Sohal R.S. Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: possible basis of variation in longevity and metabolic potential. Mech. Ageing Dev. 1993;72:67–76. doi: 10.1016/0047-6374(93)90132-B. [DOI] [PubMed] [Google Scholar]
- 31.Sohal R.S., Ku H.H., Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem. Biophys. Res. Commun. 1993;196:7–11. doi: 10.1006/bbrc.1993.2208. [DOI] [PubMed] [Google Scholar]
- 32.Herrero A., Barja G. H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech. Ageing Dev. 1998;103:133–146. doi: 10.1016/S0047-6374(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 33.Herrero A., Barja G. Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon. Mech. Ageing Dev. 1997;98:95–111. doi: 10.1016/S0047-6374(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 34.Herrero A., Barja G. ADP-regulation of mitochondrial free radical production is different with complex I-or complex II-linked substrates: implications for the exercise paradox and brain hypermetabolism. J. Bioenerg. Biomembr. 1997;29:241–249. doi: 10.1023/A:1022458010266. [DOI] [PubMed] [Google Scholar]
- 35.Barja G., Herrero A. Localization at complex I and mechanism of the higher free radical production of brain nonsynaptic mitochondria in the short-lived rat than in the longevous pigeon. J. Bioenerg. Biomembr. 1998;30:235–243. doi: 10.1023/A:1020592719405. [DOI] [PubMed] [Google Scholar]
- 36.Barja G., Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- 37.Adelman R., Saul R.L., Ames B.N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc. Natl. Acad. Sci. U S A. 1988;85:2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano M., Gotoh S. Accumulation of cardiac lipofuscin depends on metabolic rate of mammals. J. Gerontol. 1992;47:B126–B129. doi: 10.1093/geronj/47.4.b126. [DOI] [PubMed] [Google Scholar]
- 39.Sohal R.S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harman D. Aging and disease: extending functional life span. Ann. N. Y. Acad. Sci. 1996;786:321–336. doi: 10.1111/j.1749-6632.1996.tb39074.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu B.P. Aging and oxidative stress: modulation by dietary restriction. Free Radic. Biol. Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 42.Merry B.J. Calorie restriction and age-related oxidative stress. Ann. N. Y. Acad. Sci. 2000;908:180–198. doi: 10.1111/j.1749-6632.2000.tb06646.x. [DOI] [PubMed] [Google Scholar]
- 43.Sohal R.S., Ku H.H., Agarwal S., Forster M.J., Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 44.Kapahi P., Boulton M.E., Kirkwood T.B. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic. Biol. Med. 1999;26:495–500. doi: 10.1016/S0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- 45.Ishii N., Fujii M., Hartman P.S., Tsuda M., Yasuda K., Senoo-Matsuda N., Yanase S., Ayusawa D., Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- 46.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J. Bioenerg. Biomembr. 1999;31:347–366. doi: 10.1023/A:1005427919188. [DOI] [PubMed] [Google Scholar]
- 47.Barja G. Mitochondrial free radical production and aging in mammals and birds. Ann. N. Y. Acad. Sci. 1998;854:224–238. doi: 10.1111/j.1749-6632.1998.tb09905.x. [DOI] [PubMed] [Google Scholar]
- 48.Sohal, R.S., Agarwal, A., Agarwal, S. and Orr, W.C. Simultaneous overexpression of copper-and zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem. 270:15671–15674, 1995. [DOI] [PubMed]
- 49.Longo, V.D., Gralla, E.B. and Valentine, J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 271:12275–12280, 1996. [DOI] [PubMed]
- 50.Park, J.l., Grant, C.M., Davies, M.J. and Dawes, I.W. The cytoplasmic Cu,Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress. Generation of free radicals during freezing and thawing. J. Biol. Chem. 273:22921–22928, 1998. [DOI] [PubMed]
- 51.Lynch R., Fridovich I. Penetration of the erythrocyte stroma by O2−. J. Biol. Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- 52.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 53.Harman D. The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 54.Guidot, D.M., McCord, J.M., Wright, R.M. and Repine, J.E. Absence of electron transport (Rho 0 state) restores growth of a manganese-superoxide dismutase-deficient Saccharomyces cerevisiae in hyperoxia. Evidence for electron transport as a major source of superoxide generation in vivo. J. Biol. Chem. 268:26699-26703, 1993. [PubMed]
- 55.Sohal R.S. Mitochondria generate superoxide anion radicals and hydrogen peroxide. FASEB J. 1997;11:1269–1270. doi: 10.1096/fasebj.11.14.9409545. [DOI] [PubMed] [Google Scholar]
- 56.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 57.Ernster L., Nohl H., Orrenius S. How best to ameliorate the normal increase in mitochondrial superoxide formation with advancing age. Ann. N. Y. Acad. Sci. 1998;854:251–267. doi: 10.1111/j.1749-6632.1998.tb09907.x. [DOI] [PubMed] [Google Scholar]
- 58.Babior, B.M. The production and use of reactive oxygen species by phagocytes. In: Halliwell, B. and Gutteridge, J.M.C., Free radicals in Biology and Medicine, Third Edition, p. 504, 1999.
- 59.de Grey A.D. Biologists abandon Popper at their peril. BioEssays. 2000;22:206–207. doi: 10.1002/(SICI)1521-1878(200002)22:2<206::AID-BIES14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 60.Carlsson L.M., Jonsson J., Edlund T., Marklund S.L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. U S A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matzuk M.M., Dionne L., Guo Q., Kumar T.R., Lebovitz R.M. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/en.139.9.4008. [DOI] [PubMed] [Google Scholar]
- 62.Shefner J.M., Reaume A.G., Flood D.G., Scott R.W., Kowall N.W., Ferrante R.J., Siwek D.F., Upton-Rice M., Brown R.H., Jr. Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology. 1999;53:1239–1246. doi: 10.1212/wnl.53.6.1239. [DOI] [PubMed] [Google Scholar]
- 63.Ohlemiller K.K., McFadden S.L., Ding D.L., Flood D.G., Reaume A.G., Hoffman E.K., Scott R.W., Wright J.S., Putcha G.V., Salvi R.J. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol. Neurootol. 1999;4:237–246. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- 64.Forman H.J., Azzi A. On the virtual existence of superoxide anions in mitochondria: thoughts regarding its role in pathophysiology. FASEB J. 1997;11:374–375. doi: 10.1096/fasebj.11.5.9141504. [DOI] [PubMed] [Google Scholar]
- 65.Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 66.Hansford R.G., Hogue B.A., Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997;29:89–95. doi: 10.1023/A:1022420007908. [DOI] [PubMed] [Google Scholar]
- 67.Longo V.D., Liou L.L., Valentine J.S., Gralla E.B. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch. Biochem. Biophys. 1999;365:131–142. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- 68.Dutton P.L., Moser C.C., Sled V.D., Daldal F., Ohnishi T. A reductant-induced oxidation mechanism for complex I. Biochim. Biophys. Acta. 1998;1364:245–257. doi: 10.1016/S0005-2728(98)00031-0. [DOI] [PubMed] [Google Scholar]
- 69.The Complex I Homepage http://www.scripps.edu/mem/biochem/CI/index.html. 2000. The Scripps Research Institute.
- 70.Ackrell B.A. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 2000;466:1–5. doi: 10.1016/S0014-5793(99)01749-4. [DOI] [PubMed] [Google Scholar]
- 71.Crofts A.R., Wang Z. How rapid are the internal reactions of the ubiquinol: cytochrome c2 oxidoreductase? Photosynth. Res. 1989;22:69–87. doi: 10.1007/BF00114768. [DOI] [PubMed] [Google Scholar]
- 72.Michel, H. Cytochrome c oxidase: catalytic cycle and mechanisms of proton pumping— a discussion. Biochemistry 38:15129–15140, 1999. [DOI] [PubMed]
- 73.Proshlyakov D.A., Pressler M.A., Babcock G.T. Dioxygen activation and bond cleavage by mixed-valence cytochrome c oxidase. Proc. Natl. Acad. Sci. U S A. 1998;95:8020–8025. doi: 10.1073/pnas.95.14.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cramer W.A., Knaff D.B. Energy Transduction in Biological Membranes. New York: Springer-Verlag; 1989. pp. 35–74. [Google Scholar]
- 75.Wood P.M. The potential diagram for oxygen at pH 7. Biochem. J. 1988;253:287–289. doi: 10.1042/bj2530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petlicki J., Van de Ven T.G.M. The equlibrium between the oxidation of hydrogen peroxide by oxygen and the dismutation of peroxyl or superoxide radicals in aqueous solutions in contact with oxygen. Journal of the Chemical Society Faraday Transactions. 1998;94:2763–2767. doi: 10.1039/a804551h. [DOI] [Google Scholar]
- 77.Cammack R., Barber M.J., Bray R.C. Oxidation-reduction potentials of molybdenum, flavin and iron-sulphur centres in milk xanthine oxidase. Biochem. J. 1976;157:469–478. doi: 10.1042/bj1570469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buettner G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 79.Isogai Y., lizuka T., Makino R., Iyanagi T., Orii Y. Superoxide-producing cytochrome b. Enzymatic and electron paramagnetic resonance properties of cytochrome b558 purified from neutrophils. J. Biol. Chem. 1993;268:4025–4031. [PubMed] [Google Scholar]
- 80.Halliwell, B. and Gutteridge, J.M.C., Free radicals in Biology and Medicine. Third ed: Oxford University Press 1999.
- 81.Cadenas E., Boveris A., Ragan C.I., Stoppani A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 82.Turrens J.F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loschen G., Azzi A. On the formation of hydrogen peroxide and oxygen radicals in heart mitochondria. Recent Adv. Stud. Cardiac. Struct. Metab. 1976;7:3–12. [PubMed] [Google Scholar]
- 84.Hinkle P.C., Butow R.A., Racker E., Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J. Biol. Chem. 1967;242:5169–5473. [PubMed] [Google Scholar]
- 85.Imlay, J.A. A metabolic enzyme that rapidly produces superoxide, fumarate reductase of Escherichia coil J. Biol. Chem. 270:19767–19777, 1995. [PubMed]
- 86.Forman J.H., Kennedy J. Superoxide production and electron transport in mitochondrial oxidation of dihydroorotic acid. J. Biol. Chem. 1975;250:4322–4326. [PubMed] [Google Scholar]
- 87.Bolter C.J., Chefurka W. Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibitors phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes. Arch. Biochem. Biophys. 1990;278:65–72. doi: 10.1016/0003-9861(90)90232-N. [DOI] [PubMed] [Google Scholar]
- 88.Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv. Exp. Med. Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- 89.Guillivi C., Boveris A., Cadenas E. The Steady state concentration of Oxygen free radicals in Mitochondria. In: Daniel L., Gilbert C.A.C., editors. Reactive Oxygen species in Biological systems. New York: Kluwer Academic/ Plenum Publishers; 1999. pp. 77–102. [Google Scholar]
- 90.Nohl H. Is redox-cycling ubiquinone involved in mitochondrial oxygen activation? Free Radic. Res. Commun. 1990;8:307–315. doi: 10.3109/10715769009053364. [DOI] [PubMed] [Google Scholar]
- 91.Nohl H., Gille L., Schonheit K., Liu Y. Conditions allowing redox-cycling ubisemiquinone in mitochondria to establish a direct redox couple with molecular oxygen. Free. Radic. Biol. Med. 1996;20:207–213. doi: 10.1016/0891-5849(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 92.Nohl H., Jordan W. The mitochondrial site of superoxide formation. Biochem. Biophys. Res. Commun. 1986;138:533–539. doi: 10.1016/S0006-291X(86)80529-0. [DOI] [PubMed] [Google Scholar]
- 93.Nohl H., Stolze K. Ubisemiquinones of the mitochondrial respiratory chain do not interact with molecular oxygen. Free Radic. Res. Commun. 1992;16:409–419. doi: 10.3109/10715769209049191. [DOI] [PubMed] [Google Scholar]
- 94.Tyler D. The Mitochondrion in health and disease. New York: VCH; 1992. pp. 172–173. [Google Scholar]
- 95.Williams J.N. A Comparative study of cytochrome ratios in mitochondria of the rat, chicken and guinea pig. Biochim. Biophys. Acta. 1968;162:175–181. doi: 10.1016/0005-2728(68)90100-X. [DOI] [PubMed] [Google Scholar]
- 96.Iwata S., Lee J.W., Okada K., Lee J.K., Iwata M., Rasmussen B., Link T.A., Ramaswamy S., Jap B.K. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z., Huang L., Shulmeister V.M., Chi Y.I., Kim K.K., Hung L.W., Crofts A.R., Berry E.A., Kim S.H. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 98.Bowyer, J.R. and Ohnishi, T. EPR spectroscopy in the study of ubisemiquinones in redox chains. Coenzyme Q (G. Lenaz ed.) pp 409–432, 1985.
- 99.Kraut J. How do enzymes work? Science. 1988;242:533–540. doi: 10.1126/science.3051385. [DOI] [PubMed] [Google Scholar]
- 100.The bc1-Complex http://arc-genl.life.uiuc.edu/Bioph354/bc-complex_summary.html. 1996. University of Illinois at Urbana-Champaign.
- 101.Berry E.A., Huang L.S., Zhang Z., Kim S.H. Structure of the avian mitochondrial cytochrome bc1 complex. J. Bioenerg. Biomembr. 1999;31:177–190. doi: 10.1023/A:1005459426843. [DOI] [PubMed] [Google Scholar]
- 102.Bergström J. The EPR spectrum and orientation of cytochrome b-563 in the chloroplast thylakoid membrane. FEBS Lett. 1985;183:87–90. doi: 10.1016/0014-5793(85)80959-5. [DOI] [Google Scholar]
- 103.Berry, E.A. and Trumpower, B.L., Pathways of Electrons and Protons Through the Cytochrome bc1 Complex of the Mitochondrial Respiratory Chain, in Coenzyme Q, Lenaz, G., Editor., John Wiley & Sons Ltd. pp. 365–389, 1985.
- 104.Crofts, A.R., Hong, S., Ugulava, N., Barquera, B., Gennis, R., Guergova-Kuras, M. and Berry, E.A. Pathways for proton release during ubihydroquinone oxidation by the bc(1) complex. Proc. Natl. Acad. Sci. U S A 96: 10021–10026, 1999. [DOI] [PMC free article] [PubMed]
- 105.Hong, S., Ugulava, N., Guergova-Kuras, M. and Crofts, A.R. The energy landscape for ubihydroquinone oxidation at the Q(o) site of the bc(1) complex in Rhodobacter sphaeroides. J. Biol. Chem. 274:33931–33944, 1999. [DOI] [PubMed]
- 106.Ksenzenko M., Konstantinov A.A., Khomutov G.B., Tikhonov A.N., Ruuge E.K. Effect of electron transfer inhibitors on superoxide generation in the cytochrome bc1 site of the mitochondrial respiratory chain. FEBS Lett. 1983;155:19–24. doi: 10.1016/0014-5793(83)80200-2. [DOI] [PubMed] [Google Scholar]
- 107.Zhang, L., Yu, L. and Yu, C.A. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J. Biol. Chem. 273:33972–33976, 1998. [DOI] [PubMed]
- 108.Turrens J.F., Alexandre A., Lehninger A.L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 109.Longo V.D., Ellerby L.M., Bredesen D.E., Valentine J.S., Gralla E.B. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell. Biol. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.T’Sai A. L., Palmer G. Potentiometric studies on yeast complex III. Biochim. Biophys. Acta. 1983;722:349–363. doi: 10.1016/0005-2728(83)90083-X. [DOI] [PubMed] [Google Scholar]
- 111.Crofts, A.R., Barquera, B., Gennis, R.B., Kuras, R., Guergova-Kuras, M. and Berry, E.A. Mechanism of ubiquinol oxidation by the bc(1) complex: different domains of the quinol binding pocket and their role in the mechanism and binding of inhibitors. Biochemistry 38:15807–15826, 1999. [DOI] [PubMed]
- 112.Ding, H., Moser, C.C., Robertson, D.E., Tokito, M.K., Daldal, F. and Dutton, P.L. Ubiquinone pair in the Qo site central to the primary energy conversion reactions of cytochrome bc1 complex. Biochemistry 34: 15979–15996, 1995. [DOI] [PubMed]
- 113.Che, Y., Tsushima, M., Matsumoto, F., Okajima, T., Tokuda, K. and Ohsaka, T. Water-induced Disproportionation of Superoxide Ion in Aprotic Solvents. J. Phys. Chem. 100:20134–20137, 1996.
- 114.Sawyer D.T. Oxygen: Inorganic Chemistry. In: King R.B., editor. Encyclopedia of Inorganic Chemistry. Chichester: John Wiley & Sons; 1994. pp. 2947–2988. [Google Scholar]
-
115.Bielski B.H.J. Reactivity of
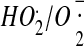 Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data. 1985;14:1041–1091. [Google Scholar]
Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data. 1985;14:1041–1091. [Google Scholar] - 116.Takahashi M.A., Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Biochem. Biophys. 1983;226:558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- 117.Turrens J.F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/A:1027374931887. [DOI] [PubMed] [Google Scholar]
- 118.Ernster L., Hoberman H.D., Howard R.L., King T.E., Lee C.P., Mackler B., Sottocasa G. Stereospecificity of certain soluble and particulate preparations of mitochondrial reduced nicotinamide-adenine dinucleotide dehydrogenase from beef heart. Nature. 1965;207:940–941. doi: 10.1038/207940a0. [DOI] [PubMed] [Google Scholar]
- 119.Parsons D.F. Recent advances correlating structure and function in mitochondria. Int. Rev. Exp. Pathol. 1965;4:1–54. [PubMed] [Google Scholar]
- 120.Lass A., Sohal R.S. Effect of coenzyme Q(10) and alpha-tocopherol content of mitochondria on the production of superoxide anion radicals. FASEB J. 2000;14:87–94. doi: 10.1096/fasebj.14.1.87. [DOI] [PubMed] [Google Scholar]
- 121.Fukuzawa K., Gebicki J.M. Oxidation of alpha-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch. Biochem. Biophys. 1983;226:242–251. doi: 10.1016/0003-9861(83)90290-4. [DOI] [PubMed] [Google Scholar]
- 122.Hoch F.L. Cardiolipins and mitochondrial proton-selective leakage. J. Bioenerg. Biomembr. 1998;30:511–532. doi: 10.1023/A:1020576315771. [DOI] [PubMed] [Google Scholar]
- 123.Korshunov S.S., Skulachev V.P., Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/S0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
-
124.Korshunov S.S., Korkina O.V., Ruuge E.K., Skulachev V.P., Starkov A.A. Fatty acids as natural uncouplers preventing generation of
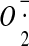 and H2O2 by mitochondria in the resting state. FEBS Lett. 1998;435:215–228. doi: 10.1016/S0014-5793(98)01073-4. [DOI] [PubMed] [Google Scholar]
and H2O2 by mitochondria in the resting state. FEBS Lett. 1998;435:215–228. doi: 10.1016/S0014-5793(98)01073-4. [DOI] [PubMed] [Google Scholar] - 125.Blasig I.E., Dickens B.F., Weglicki W.B., Kramer J.H. Uncoupling of mitochondrial oxidative phosphorylation alters lipid peroxidation-derived free radical production but not recovery of postischemic rat hearts and post-hypoxic endothelial cells. Mol. Cell. Biochem. 1996;160–161:167–177. doi: 10.1007/BF00240047. [DOI] [PubMed] [Google Scholar]
- 126.Skulachev V.P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29:169–202. doi: 10.1017/S0033583500005795. [DOI] [PubMed] [Google Scholar]
-
127.Demin O.V., Kholodenko B.N., Skulachev V.P. A model of
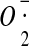 generation in the complex III of the electron transport chain. Mol. Cell. Biochem. 1998;184:21–33. doi: 10.1023/A:1006849920918. [DOI] [PubMed] [Google Scholar]
generation in the complex III of the electron transport chain. Mol. Cell. Biochem. 1998;184:21–33. doi: 10.1023/A:1006849920918. [DOI] [PubMed] [Google Scholar] - 128.Pitkanen S., Robinson B.H. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J. Clin. Invest. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tanaka M., Gong J.S., Zhang J., Yoneda M., Yagi K. Mitochondrial genotype associated with longevity. Lancet. 1998;351:185–186. doi: 10.1016/S0140-6736(05)78211-8. [DOI] [PubMed] [Google Scholar]
- 130.Takeshige K., Minakami S. NADH-and NADPH-dependent formation ol superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem. J. 1979;180:129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Herrero, A. and Barja, G. Localisation of the site of oxygen radical generation inside Complex I of heart and non-synaptic brain mammalian mitochondria. J. Bioenerg. Biomembr.: In press, 2000. [DOI] [PubMed]
- 132.Grigorieff N. Structure of the respiratory NADH:ubiquinone oxidoreductase (complex I) Curr. Opin. Struct. Biol. 1999;9:476–483. doi: 10.1016/S0959-440X(99)80067-0. [DOI] [PubMed] [Google Scholar]
- 133.Grigorieff N. Three-dimensional structure of bovine NADH:ubiquinone oxidoreductase (complex I) at 22 A in ice. J. Mol. Biol. 1998;277:1033–1046. doi: 10.1006/jmbi.1998.1668. [DOI] [PubMed] [Google Scholar]
- 134.Guenebaut V., Schlitt A., Weiss H., Leonard K., Friedrich T. Consistent structure between bacterial and mitochondriai NADH:ubiquinone oxidoreductase (complex I) J. Mol. Biol. 1998;276:105–112. doi: 10.1006/jmbi.1997.1518. [DOI] [PubMed] [Google Scholar]
- 135.Ohnishi T. Iron-sulfur clusters/semiquinones in complex I. Biochim. Biophys. Acta. 1998;1364:186–206. doi: 10.1016/S0005-2728(98)00027-9. [DOI] [PubMed] [Google Scholar]
- 136.Ohnishi T., Sled V.D., Yano T., Yagi T., Burbaev D.S., Vinogradov A.D. Structure-function studies of iron-sulfur clusters and semiquinones in the NADH-Q oxidoreductase segment of the respiratory chain. Biochim. Biophys. Acta. 1998;1365:301–308. doi: 10.1016/S0005-2728(98)00082-6. [DOI] [PubMed] [Google Scholar]
- 137.van Belzen R., Kotlyar A.B., Moon N., Dunham W.R., Albracht S.P. The iron-sulfur clusters 2 and ubisemiquinone radicals of NADH:ubiquinone oxidoreductase are involved in energy coupling in submitochondrial particles. Biochemistry. 1997;36:886–893. doi: 10.1021/bi9612982. [DOI] [PubMed] [Google Scholar]
- 138.Brandt U. Proton-translocation by membrane-bound NADPH:ubiquinone-oxidoreductase (complex I) through redox-gated ligand conduction. Biochim. Biophys. Acta. 1997;1318:79–91. doi: 10.1016/S0005-2728(96)00141-7. [DOI] [PubMed] [Google Scholar]
- 139.Degli Esposti M., Ghelli A., Crimi M., Estornell E., Fato R., Lenaz G. Complex I and complex III of mitochondria have common inhibitors acting as ubiquinone antagonists. Biochem. Biophys. Res. Commun. 1993;190:1090–1096. doi: 10.1006/bbrc.1993.1161. [DOI] [PubMed] [Google Scholar]
- 140.Carelli V., Ghelli A., Bucchi L., Montagna P., De Negri A., Leuzzi V., Carducci C., Lenaz G., Lugaresi E., Degli Esposti M. Biochemical features of mtDNA 14484 (ND6/M64V) point mutation associated with Leber’s hereditary optic neuropathy. Ann. Neurol. 1999;45:320–328. doi: 10.1002/1531-8249(199903)45:3<320::AID-ANA7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 141.Fisher N., Rich P.R. A Motif for Quinone Binding Sites in Respiratory and Photosynthetic Systems. J. Mol. Biol. 2000;296:1153–1162. doi: 10.1006/jmbi.2000.3509. [DOI] [PubMed] [Google Scholar]
- 142.Boveris A., Cadenas E., Stoppani A.O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Messner, K.R. and Imlay, J.A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coll. J. Biol. Chem. 274: 10119–10128, 1999. [DOI] [PubMed]
- 144.Tormo J.R., Gallardo T., Aragon R., Cortes D., Estornell E. Specific interactions of monotetrahydrofuranic annonaceous acetogenins as inhibitors of mitochondrial complex I. Chem. Biol. Interact. 1999;122:171–183. doi: 10.1016/S0009-2797(99)00120-9. [DOI] [PubMed] [Google Scholar]
- 145.Tormo J.R., Gonzalez M.C., Cortes D., Estornell E. Kinetic characterization of mitochondrial complex I inhibitors using annonaceous acetogenins. Arch. Biochem. Biophys. 1999;369:119–126. doi: 10.1006/abbi.1999.1343. [DOI] [PubMed] [Google Scholar]
- 146.Barja G., Cadenas S., Rojas C., Lopez-Torres M., Perez-Campo R. A decrease of free radical production near critical targets as a cause of maximum longevity in animals. Comp. Biochem. Physiol. Biochem. Mol. Biol. 1994;108:501–512. doi: 10.1016/0305-0491(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 147.de Grey A.D.N.J. Non-correlation between maximum life span and antioxidant enzyme levels among homotherms: implications for retarding human aging. J. Antiaging Med. 2000;3:25–36. [Google Scholar]
- 148.Mockett R.J., Sohal R.S., Orr W.C. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 149.Mockett R.J., Orr W.C., Rahmandar J.J., Benes J.J., Radyuk S.N., Klichko V.I., Sohal R.S. Overexpression of Mn-containing superoxide dismutase in transgenic Drosophila melanogaster. Arch. Biochem. Biophys. 1999;371:260–269. doi: 10.1006/abbi.1999.1460. [DOI] [PubMed] [Google Scholar]
- 150.Srere P.A. The Structure of the mitochondrial inner membrane-matrix compartement. Trends. Biochem. Sci. 1982;7:375–378. doi: 10.1016/0968-0004(82)90119-0. [DOI] [Google Scholar]
- 151.de Grey A.D.N.J. A proposed refinement of the mitochondrial free radical theory of aging. BioEssays. 1997;19:161–166. doi: 10.1002/bies.950190211. [DOI] [PubMed] [Google Scholar]
- 152.de Grey A.D.N.J. A mechanism proposed to explain the rise in oxidative stress during aging. J. Antiaging Med. 1998;1:53–66. [Google Scholar]
- 153.Voet D., Voet J.G. Chapter 18: Transport through membranes. First Edition. New York: John Wiley & Sons; 1990. Biochemistry. [Google Scholar]
- 154.Antunes F., Salvador A., Marinho H.S., Alves R., Pinto R.E. Lipid peroxidation in mitochondrial inner membranes. I. An integrative kinetic model. Free Radic. Biol. Med. 1996;21:917–943. doi: 10.1016/S0891-5849(96)00185-2. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi M., Asada K. A flash-photometric method for determination of reactivity of superoxide: application to superoxide dismutase assay. J. Biochem. (Tokyo) 1982;91:889–896. doi: 10.1093/oxfordjournals.jbchem.a133777. [DOI] [PubMed] [Google Scholar]
- 156.Aikens, J. and Dix, T.A. Perhydroxyl radical (HOO•) initiated lipid peroxidation. The role of fatty acid hydroperoxides. J. Biol. Chem. 266:15091–15098, 1991. [PubMed]
-
157.Thomas M.J., Sutherland M.W., Arudi R.L., Bielski B.H. Studies of the reactivity of
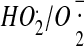 with unsaturated hydroperoxides in ethanolic solutions. Arch. Biochem. Biophys. 1984;233:772–775. doi: 10.1016/0003-9861(84)90505-8. [DOI] [PubMed] [Google Scholar]
with unsaturated hydroperoxides in ethanolic solutions. Arch. Biochem. Biophys. 1984;233:772–775. doi: 10.1016/0003-9861(84)90505-8. [DOI] [PubMed] [Google Scholar] -
158.Bielski B.H., Arudi R.L., Sutherland M.W. A study of the reactivity of
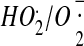 with unsaturated fatty acids. J. Biol. Chem. 1983;258:4759–4761. [PubMed] [Google Scholar]
with unsaturated fatty acids. J. Biol. Chem. 1983;258:4759–4761. [PubMed] [Google Scholar] -
159.Liochev S.I., Fridovich I. The relative importance of HO and ONOO-in mediating the toxicity of
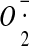 Free Radic. Biol. Med. 1999;26:777–778. doi: 10.1016/S0891-5849(98)00304-9. [DOI] [PubMed] [Google Scholar]
Free Radic. Biol. Med. 1999;26:777–778. doi: 10.1016/S0891-5849(98)00304-9. [DOI] [PubMed] [Google Scholar] - 160.Kissner R., Nauser T., Bugnon P., Lye P.G., Koppenol W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 161.Beckmann J.D., Ljungdahl P.O., Trumpower B.L. Mutational analysis of the mitochondrial Rieske iron-sulfur protein of Saccharomyces cerevisiae. I. Construction of a RIP1 deletion strain and isolation of temperature-sensitive mutants. J. Biol. Chem. 1989;264:3713–3722. [PubMed] [Google Scholar]
- 162.Lemesle-Meunier, D., Brivet-Chevillotte, P., di Rago, J.P., Slonimski, P.P., Bruel, C., Tron, T. and Forget, N. Cytochrome b-deficient mutants of the ubiquinol-cytochrome c oxidoreductase in Saccharomyces cerevisiae. Consequence for the functional and structural characteristics of the complex. J. Biol. Chem. 268: 15626–15632, 1993. [PubMed]
- 163.VidaI-Puig, A.J., Grujic, D., Zhang, C.Y., Hagen, T., Boss, O., Ido, Y., Szczepanik, A., Wade, J., Mootha, V., Cortright, R., Muoio, D.M. and Lowell, B.B. Energy metabolism in uncoupling protein 3 gene knockout mice. J. Biol. Chem. 275:16258–16266, 2000. [DOI] [PubMed]
- 164.Esposito L.A., Melov S., Panov A., Cottrell B.A., Wallace D.C. Mitochondrial disease in mouse results in increased oxidative stress. Proc. Natl. Acad. Sci. U S A. 1999;96:4820–4825. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Graham B.H., Waymire K.G., Cottrell B., Trounce I.A., MacGregor G.R., Wallace D.C. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat. Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 166.Taylor R.W., Birch-Machin M.A., Bartlett K., Lowerson S.A., Turnbull D.M. The control of mitochondrial oxidations by complex III in rat muscle and liver mitochondria. Implications for our understanding of mitochondrial cytopathies in man. J. Biol. Chem. 1994;269:3523–3528. [PubMed] [Google Scholar]
- 167.Boveds A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Baret P., Fouarge A., Bullens P., Lints F.A. Life-span of Drosophila melanogaster in highly oxygenated atmospheres. Mech. Ageing Dev. 1994;76:25–31. doi: 10.1016/0047-6374(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 169.Honda S., Matsuo M. Lifespan shortening of the nematode Caenorhabditis elegans under higher concentrations of oxygen. Mech. Ageing Dev. 1992;63:235–246. doi: 10.1016/0047-6374(92)90002-U. [DOI] [PubMed] [Google Scholar]
- 170.Honda S., Ishii N., Suzuki K., Matsuo M. Oxygen-dependent perturbation of life span and aging rate in the nematode. J. Gerontol. 1993;48:B57–61. doi: 10.1093/geronj/48.2.b57. [DOI] [PubMed] [Google Scholar]
- 171.Miquel J., Lundgren P.R., Bensch K.G. Effects of oxygen-nitrogen (1:1) at 760 Torr on the life span and fine structure of Drosophila melanogaster. Mech. Ageing. Dev. 1975;4:41–57. doi: 10.1016/0047-6374(75)90006-8. [DOI] [PubMed] [Google Scholar]
- 172.Strehler B.L. Time, Cells, and Aging. 2nd Edition. New York: Academic Press; 1977. [Google Scholar]
- 173.Holmes D.J., Austad S.N. Birds as animal models for the comparative biology of aging: a prospectus. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50:B59–66. doi: 10.1093/gerona/50a.2.b59. [DOI] [PubMed] [Google Scholar]
- 174.Austad S.N., Fischer K.E. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 1991;46:B47–53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- 175.Ooka H., Shinkai T. Effects of chronic hyperthyroidism on the lifespan of the rat. Mech. Ageing Dev. 1986;33:275–282. doi: 10.1016/0047-6374(86)90052-7. [DOI] [PubMed] [Google Scholar]
- 176.Hunter W.S., Croson W.B., Bartke A., Gentry M.V., Meliska C.J. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol. Behav. 1999;67:433–437. doi: 10.1016/S0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 177.Bartke A., Brown-Borg H.M., Bode A.M., Carlson J., Hunter W.S., Bronson R.T. Does growth hormone prevent or accelerate aging? Exp. Gerontol. 1998;33:675–687. doi: 10.1016/S0531-5565(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 178.Sohal R.S., Sohal B.H. Hydrogen peroxide release by mitochondria increases during aging. Mech. Ageing. Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-W. [DOI] [PubMed] [Google Scholar]
- 179.Nishiki K., Erecinska M., Wilson D.F., Cooper S. Evaluation of oxidative phosphorylation in hearts from euthyroid, hypothyroid, and hyperthyroid rats. Am. J. Physiol. 1978;235:C212–219. doi: 10.1152/ajpcell.1978.235.5.C212. [DOI] [PubMed] [Google Scholar]
- 180.Ljungdahl P.O., Pennoyer J.D., Robertson D.E., Trumpower B.L. Purification of highly active cytochrome bc1 complexes from phylogenetically diverse species by a single chromatographic procedure. Biochim. Biophys. Acta. 1987;891:227–241. doi: 10.1016/0005-2728(87)90218-0. [DOI] [PubMed] [Google Scholar]
- 181.Braidot E., Petrussa E., Vianello A., Macri F. Hydrogen peroxide generation by higher plant mitochondria oxidizing complex I or complex II substrates. FEBS Lett. 1999;451:347–350. doi: 10.1016/S0014-5793(99)00616-X. [DOI] [PubMed] [Google Scholar]
- 182.Maxwell D.P., Wang Y., McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. U S A. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yukioka H., Inagaki S., Tanaka R., Katoh K., Miki N., Mizutani A., Masuko M. Transcriptional activation of the alternative oxidase gene of the fungus Magnaporthe grisea by a respiratory-inhibiting fungicide and hydrogen peroxide. Biochim. Biophys. Acta. 1998;1442:161–169. doi: 10.1016/s0167-4781(98)00159-6. [DOI] [PubMed] [Google Scholar]
- 184.Popov V.N., Simonian R.A., Skulachev V.P., Starkov A.A. Inhibition of the alternative oxidase stimulates H2O2 production in plant mitochondria. FEBS Lett. 1997;415:87–90. doi: 10.1016/S0014-5793(97)01099-5. [DOI] [PubMed] [Google Scholar]
- 185.Clapham J.C., Arch J.R., Chapman H., Haynes A., Lister C., Moore G.B., Piercy V., Carter S.A., Lehner I., Smith S.A., Beeley L.J., Godden R.J., Herrity N., Skehel M., Changani K.K., Hockings P.D., Reid D.G., Squires S.M., Hatcher J., Trail B., Latcham J., Rastan S., Harper A.J., Cadenas S., Buckingham J.A., Brand M.D., Abuin A. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- 186.Trumpower, B.L. The Protonmotive Q Cycle. J Biol Chem 265:11409–11412 1990 [PubMed]
- 187.Dutton P.L., Wilson D.F. Redox potentiometry in mitochondrial and photosynthetic bioenergetics. Biochim. Biophys. Acta. 1974;346:165–212. doi: 10.1016/0304-4173(74)90008-1. [DOI] [PubMed] [Google Scholar]
- 188.Wilson D.F., Dutton P.L. The oxidation-reduction potentials of cytochromes a and a3 in intact rat liver mitochondria. Arch. Biochem. Biophys. 1970;136:583–585. doi: 10.1016/0003-9861(70)90233-X. [DOI] [PubMed] [Google Scholar]


