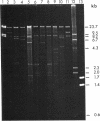
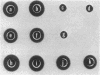
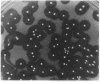
Abstract
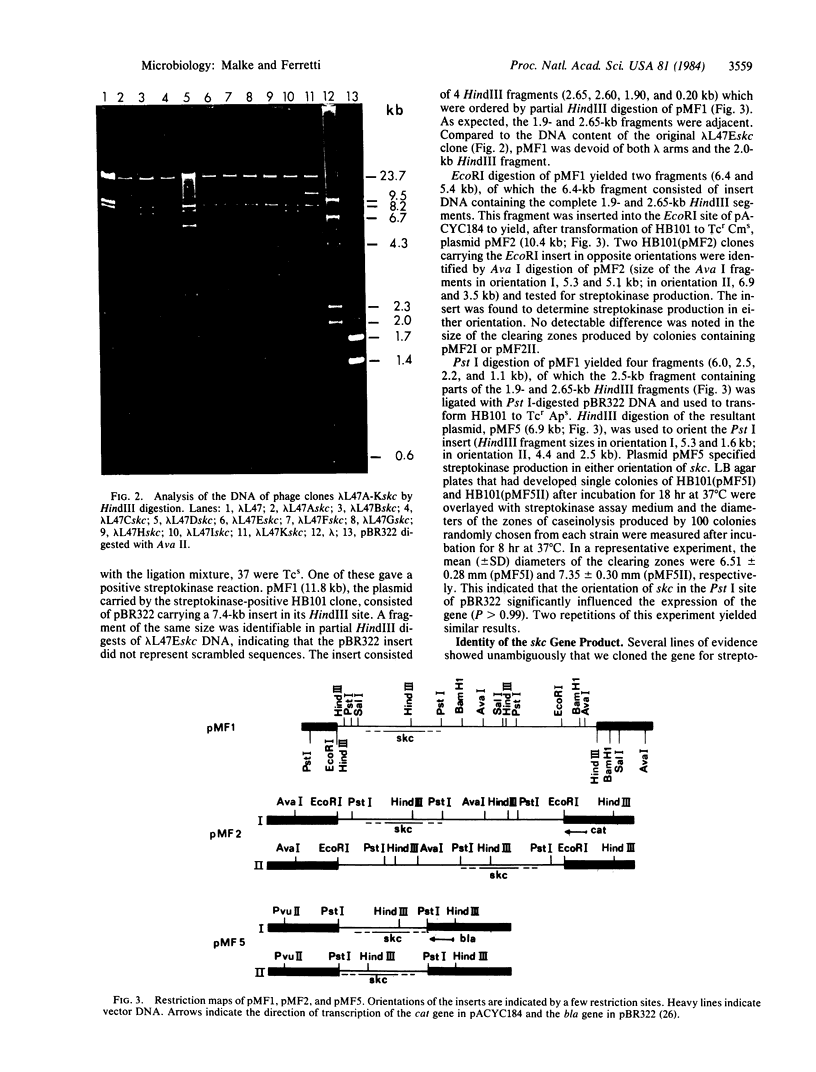
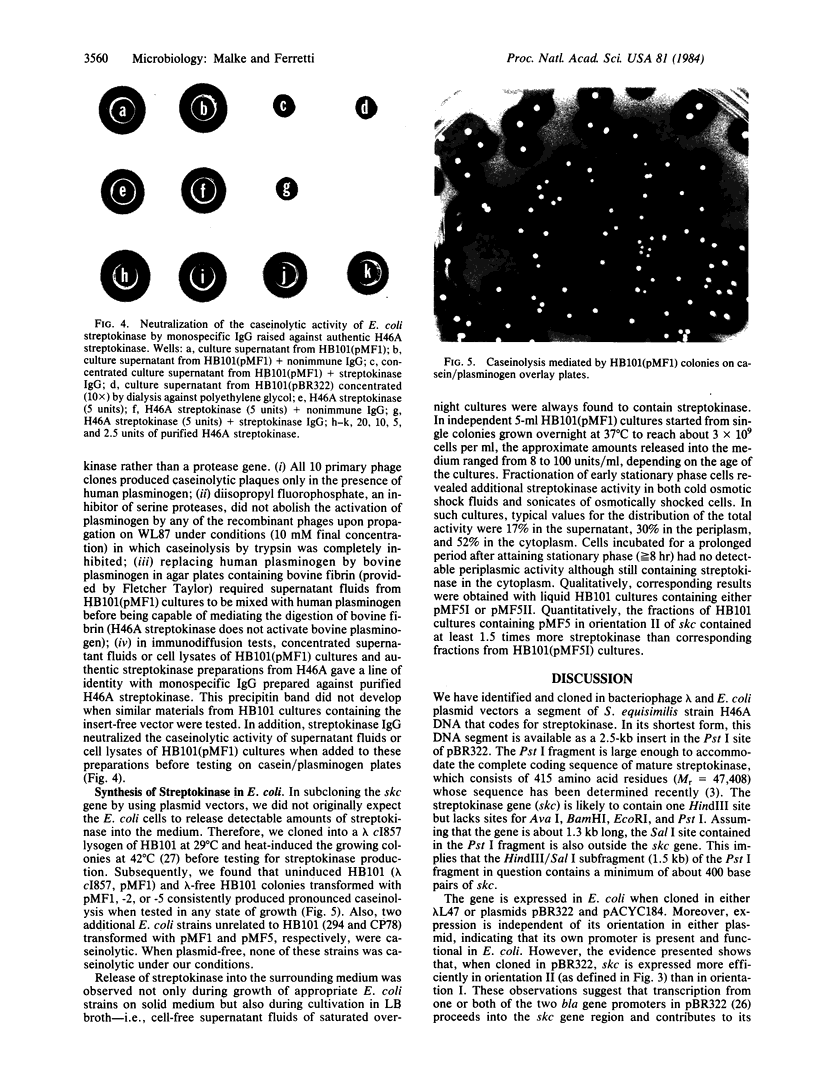
Genomic DNA from Streptococcus equisimilis strain H46A was cloned in Escherichia coli by using the bacteriophage lambda replacement vector L47 and an in vitro packaging system. A casein/plasminogen overlay technique was used to screen the phage bank for recombinants carrying the streptokinase gene ( skc ). The gene was present with a frequency of 1 in 836 recombinants, and 10 independent clones containing skc were isolated and physically characterized. One recombinant clone was used to subclone skc in E. coli plasmid vectors. Plasmid pMF2 [10.4 kilobases (kb)] consisting of pACYC184 with a 6.4-kb H46A DNA fragment in the EcoRI site and pMF5 (6.9 kb) carrying a 2.5-kb fragment in the Pst I site of pBR322 were among the recombinant plasmids determining streptokinase production in three different E. coli host strains. Expression of skc was independent of its orientation in either vector, indicating that its own promoter was present and functional in E. coli. However, expression in pBR322 was more efficient in one orientation than in the other, suggesting that one or both of the bla gene promoters contributed to skc expression. Several lines of evidence, including proof obtained by the immunodiffusion technique, established the identity of E. coli streptokinase. Testing cell-free culture supernatant fluids, osmotic shock fluids, and sonicates of osmotically shocked cells for streptokinase activity revealed the substance to be present in all three principal locations, indicating that E. coli cells were capable of releasing substantial amounts of streptokinase into the culture medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- DILLON H. C., Jr, WANNAMAKER L. W. PHYSICAL AND IMMUNOLOGICAL DIFFERENCES AMONG STREPTOKINASES. J Exp Med. 1965 Mar 1;121:351–371. doi: 10.1084/jem.121.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Gerlach D., Köhler W. Untersuchungen zur Heterogenität von Streptokinasen IV. Mitteilung: Der Nachweis von Isostreptokinasen bei Streptococcus pyogenes Typ 1. Zentralbl Bakteriol A. 1981 Feb;248(4):446–454. [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K. W., Tang J. Complete amino acid sequence of streptokinase and its homology with serine proteases. Biochemistry. 1982 Dec 21;21(26):6620–6625. doi: 10.1021/bi00269a001. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Malke H., Jacob H. E., Störl K. Characterization of the antibiotic resistance plasmid ERL1 from Streptococcus pyogenes. Mol Gen Genet. 1976 Mar 30;144(3):333–338. doi: 10.1007/BF00341732. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Lee S. G., Schrenk W. J., Roychoudhury R., Chen M., Hamilton T. A., Hung P. P. Expression in Escherichia coli of biologically active enzyme by a DNA sequence coding for the human plasminogen activator urokinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3313–3317. doi: 10.1073/pnas.78.6.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako T., Sawaki S., Sakurai T., Ito S., Yoshizawa Y., Kondo I. Cloning and expression of the staphylokinase gene of Staphylococcus aureus in Escherichia coli. Mol Gen Genet. 1983;190(2):271–277. doi: 10.1007/BF00330650. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Fischetti V. A. Expression of streptococcal M protein in Escherichia coli. Science. 1983 Aug 19;221(4612):758–760. doi: 10.1126/science.6192499. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. A comprehensive molecular map of bacteriophage lambda. Gene. 1979 Nov;7(3-4):217–270. doi: 10.1016/0378-1119(79)90047-7. [DOI] [PubMed] [Google Scholar]