Abstract
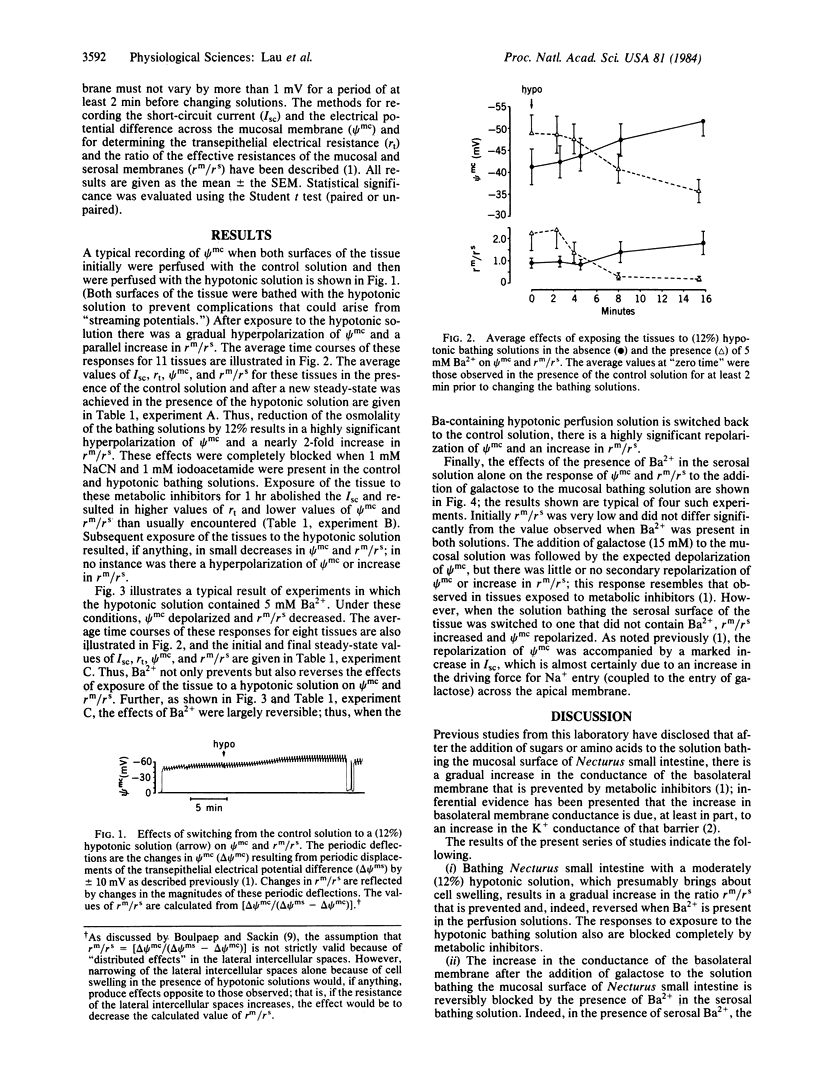
Previous studies have shown that, immediately after the addition of galactose or alanine to the solution bathing the mucosal surface of Necturus small intestine, there is a rapid depolarization of the electrical potential difference across the mucosal membrane (psi mc). This is followed by a repolarization of psi mc that is paralleled by an increase in the ratio of the effective resistance of the mucosal membrane to that of the basolateral membrane (rm/rs); the latter was shown to be, at least in part, due to a marked increase in the conductance of the basolateral membrane. We now report the following. (i) Exposure of this epithelium to a 12% hypotonic solution results in a hyperpolarization of psi mc and an increase in rm/rs. These effects are blocked by metabolic inhibitors and by the presence of 5 mM Ba2+ in the bathing solution; indeed, in the presence of Ba2+, psi mc depolarizes and rm/rs decreases to low values. (ii) Addition of 15 mM galactose to the mucosal solution when the serosal solution alone contains 5 mM Ba2+ results in a depolarization of psi mc but no subsequent repolarization of psi mc or increase in rm/rs; however, psi mc repolarizes and rm/rs increases when Ba2+ is subsequently removed from the serosal bathing solution. We conclude that (i) the basolateral membrane normally possesses a Ba2+-inhibitable K conductance, which appears to be reduced in the presence of metabolic inhibitors; (ii) after exposure of the tissue to a hypotonic solution or the addition of galactose to the mucosal solution, this conductance increases; and (iii) these responses can be blocked by metabolic inhibitors. These findings suggest that the delayed response of this tissue to the addition of sugars or amino acids to the mucosal solution may be the result of cell swelling resulting from the intracellular accumulation of these solutes in osmotically active forms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Swenson R. P., Jr, Taylor S. R. Block of squid axon K channels by internally and externally applied barium ions. J Gen Physiol. 1982 Nov;80(5):663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Taylor S. R. Interaction of barium ions with potassium channels in squid giant axons. Biophys J. 1980 Jun;30(3):473–488. doi: 10.1016/S0006-3495(80)85108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. M., Musselman D. L., Reitzug H. C. Sodium, potassium, and water content of isolated bullfrog small intestinal epithelia. Am J Physiol. 1970 Oct;219(4):1023–1026. doi: 10.1152/ajplegacy.1970.219.4.1023. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csáky T. Z., Esposito G. Osmotic swelling of intestinal epithelial cells during active sugar transport. Am J Physiol. 1969 Sep;217(3):753–755. doi: 10.1152/ajplegacy.1969.217.3.753. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Sodium transport inhibition by amiloride reduces basolateral membrane potassium conductance in tight epithelia. Science. 1982 Apr 30;216(4545):525–527. doi: 10.1126/science.7071599. [DOI] [PubMed] [Google Scholar]
- Dellasega M., Grantham J. J. Regulation of renal tubule cell volume in hypotonic media. Am J Physiol. 1973 Jun;224(6):1288–1294. doi: 10.1152/ajplegacy.1973.224.6.1288. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Reuss L. Effects of changes in the composition of the serosal solution on the electrical properties of the toad urinary bladder epithelium. J Physiol. 1975 Sep;250(3):541–558. doi: 10.1113/jphysiol.1975.sp011069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Grasset E., Schultz S. G. Sodium-coupled amino acid and sugar transport by Necturus small intestine. An equivalent electrical circuit analysis of a rheogenic co-transport system. J Membr Biol. 1982;66(1):25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Halm D. R., Dawson D. C. Active sodium transport by turtle colon via an electrogenic Na-K exchange pump. Nature. 1980 Sep 18;287(5779):237–239. doi: 10.1038/287237a0. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Effect of amiloride, ouabain and Ba++ on the nonsteady-state Na - K pump flux and short-circuit current in isolated frog skin epithelia. J Membr Biol. 1982;65(3):227–234. doi: 10.1007/BF01869966. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Fuisz R. E., Curran P. F. Amino acid and sugar transport in rabbit ileum. J Gen Physiol. 1966 May;49(5):849–866. doi: 10.1085/jgp.49.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Sodium-coupled solute transport of small intestine: a status report. Am J Physiol. 1977 Oct;233(4):E249–E254. doi: 10.1152/ajpendo.1977.233.4.E249. [DOI] [PubMed] [Google Scholar]
- Thomas S. R., Suzuki Y., Thompson S. M., Schultz S. G. Electrophysiology of Necturus urinary bladder: I. "Instantaneous" current-voltage relations in the presence of varying mucosal sodium concentrations. J Membr Biol. 1983;73(2):157–175. doi: 10.1007/BF01870439. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Barium inhibition of basolateral membrane potassium conductance in tracheal epithelium. Am J Physiol. 1983 Jun;244(6):F639–F645. doi: 10.1152/ajprenal.1983.244.6.F639. [DOI] [PubMed] [Google Scholar]


