Abstract
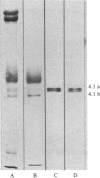
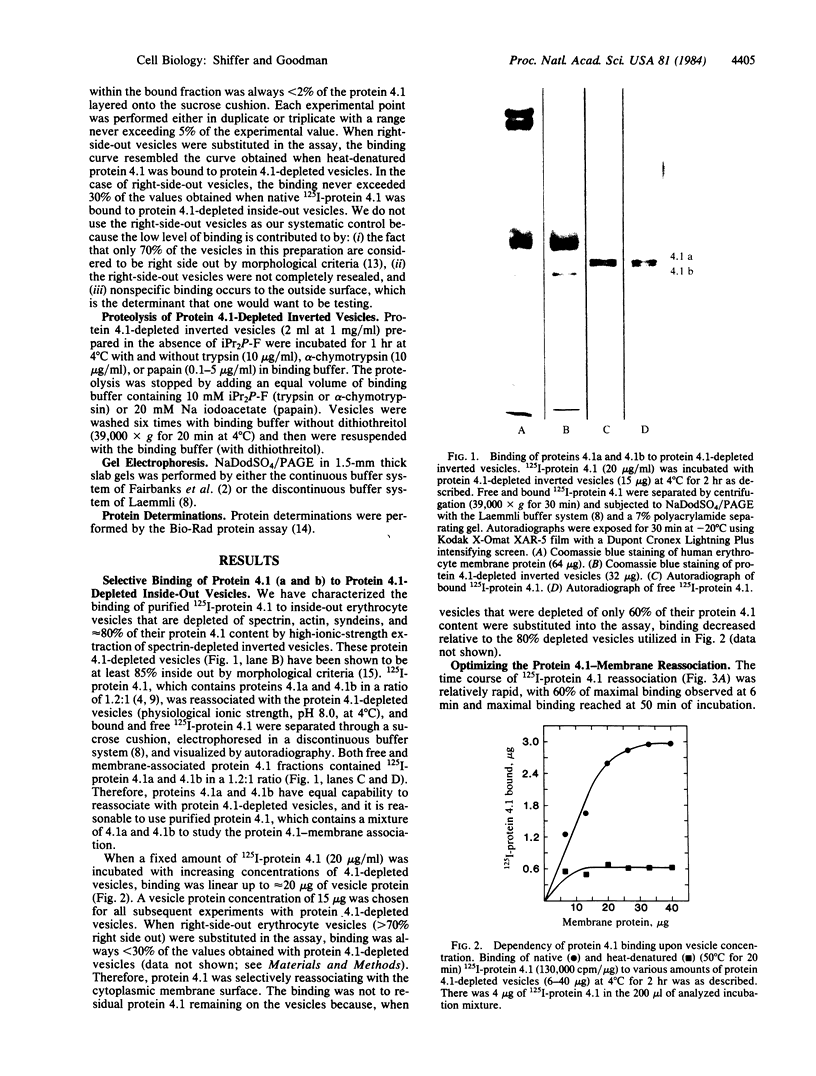
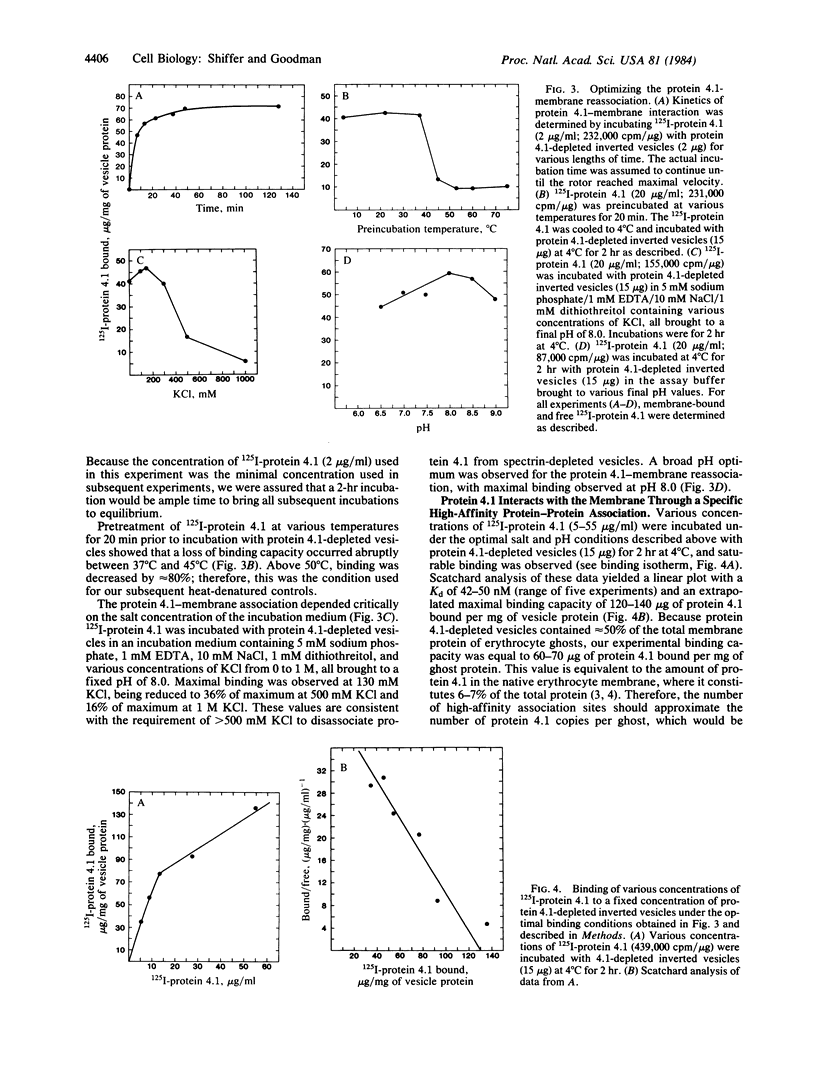
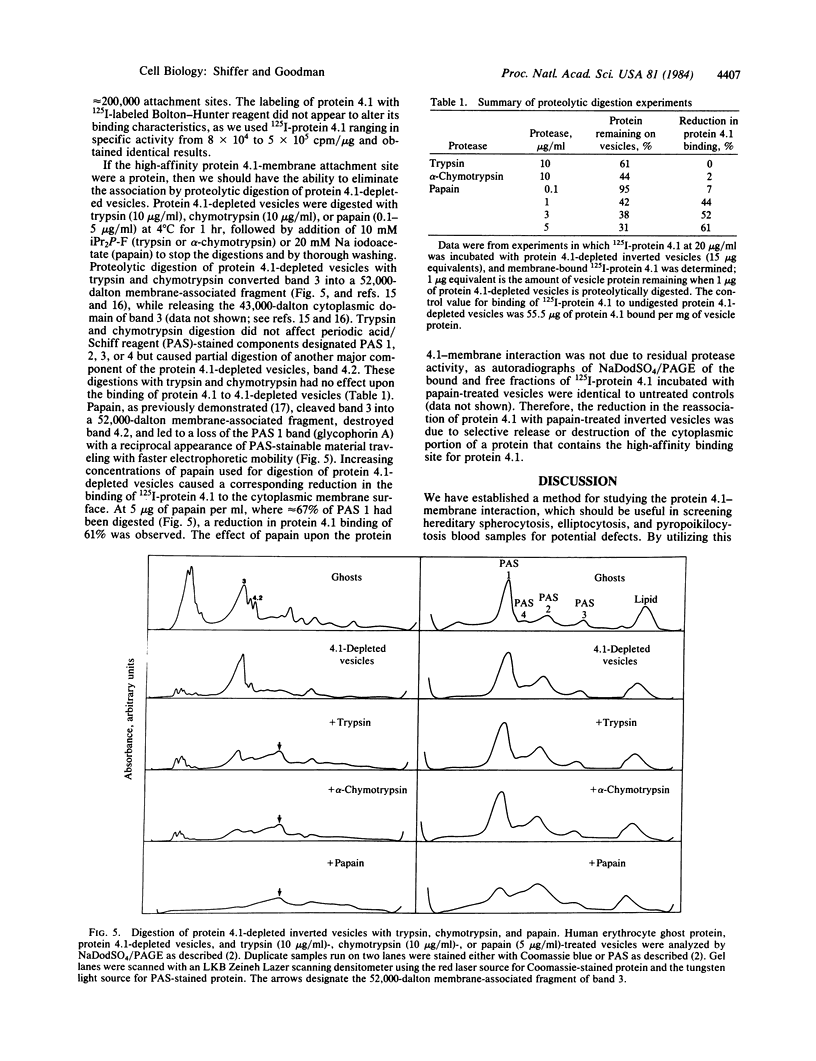
125I-labeled protein 4.1a and 4.1b have equal ability to reassociate with inside-out erythrocyte vesicles that were depleted of protein 4.1 in addition to other peripheral membrane proteins. The reassociation of 125I-labeled protein 4.1 to protein 4.1-depleted vesicles at 4 degrees C is salt dependent, pH dependent, and saturable with a Kd of 42-50 nM and an extrapolated maximal binding capacity of 120-140 micrograms of protein 4.1 bound per mg of vesicle protein or 60-70 micrograms of protein 4.1 bound per mg of ghost protein, correlating with the protein 4.1 content in the erythrocyte membrane (6-7% of the total membrane protein). Selective proteolytic cleavage of these vesicles with papain (5 micrograms/ml at 4 degrees C) eliminates greater than 60% of the high-affinity binding sites; therefore, we conclude that the interaction of protein 4.1 with the cytoplasmic membrane surface is through a specific high-affinity protein-protein association.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F. The role of band 4.1 in the association of actin with erythrocyte membranes. Biochim Biophys Acta. 1982 Jun 28;688(3):691–701. doi: 10.1016/0005-2736(82)90281-4. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Glycophorins A, B, and C: a family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J Supramol Struct. 1978;9(1):79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. A., Casoria L. A., Eyster M. E. Identification of the molecular defect in the erythrocyte membrane skeleton of some kindreds with hereditary spherocytosis. Blood. 1982 Sep;60(3):772–784. [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. The spectrin membrane skeleton of normal and abnormal human erythrocytes: a review. Am J Physiol. 1983 Mar;244(3):C121–C141. doi: 10.1152/ajpcell.1983.244.3.C121. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Yu J., Whitfield C. F., Culp E. N., Posnak E. J. Erythrocyte membrane skeletal protein bands 4.1 a and b are sequence-related phosphoproteins. J Biol Chem. 1982 Apr 25;257(8):4564–4569. [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Bron C., Girardet M., Cherry R. J. Band 3-glycophorin A association in erythrocyte membrane demonstrated by combining protein diffusion measurements with antibody-induced cross-linking. Biochemistry. 1980 Apr 29;19(9):1887–1893. doi: 10.1021/bi00550a024. [DOI] [PubMed] [Google Scholar]
- Sato S. B., Ohnishi S. Interaction of a peripheral protein of the erythrocyte membrane, band 4.1, with phosphatidylserine-containing liposomes and erythrocyte inside-out vesicles. Eur J Biochem. 1983 Jan 17;130(1):19–25. doi: 10.1111/j.1432-1033.1983.tb07111.x. [DOI] [PubMed] [Google Scholar]
- Shohet S. B. Reconstitution of spectrin-deficient, spherocytic mouse erythrocyte membranes. J Clin Invest. 1979 Aug;64(2):483–494. doi: 10.1172/JCI109486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- Yu J., Steck T. L. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J Biol Chem. 1975 Dec 10;250(23):9170–9175. [PubMed] [Google Scholar]