Abstract
The micrococcal nuclease cleavage of intact nuclear chromatin from African green monkey cells and of the completely deproteinized sequences was studied by using high-resolution analytical and DNA sequencing gels and secondary restriction enzyme analysis. When deproteinized component alpha DNA was used as substrate, not all phosphodiester bonds in the 172-base-pair repeat units were cleaved with equal frequency by the nuclease. A distinct preference for the cleavage of A-T rather than G-C bonds was observed; however, A + T-richness in itself did not confer susceptibility to cleavage by micrococcal nuclease. The results suggested that, in deproteinized DNA, nuclease cleavage at particular dinucleotides may be influenced more by the effect of adjacent sequences than by the composition of the dinucleotide. In contrast to complex cleavage patterns of the deproteinized component alpha DNA which arose because of multiple cleavage sites in the repeat unit, micrococcal nuclease cleaved component alpha nuclear chromatin at one site per nucleosome repeat, near position 126 in the nucleotide sequence. This simple chromatin cleavage pattern is consistent with the discrete nucleosomal structure of component alpha in chromatin and a direct phase relationship between the component alpha DNA sequence repeats and the nucleosome protein structural repeats.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Gannon F., Chambon P. Nucleosome structure III: the structure and transcriptional activity of the chromatin containing the ovalbumin and globin genes in chick oviduct nuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):779–791. doi: 10.1101/sqb.1978.042.01.078. [DOI] [PubMed] [Google Scholar]
- Bodell W. J., Cleaver J. E. Transient conformation changes in chromatin during excision repair of ultraviolet damage to DNA. Nucleic Acids Res. 1981 Jan 10;9(1):203–213. doi: 10.1093/nar/9.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
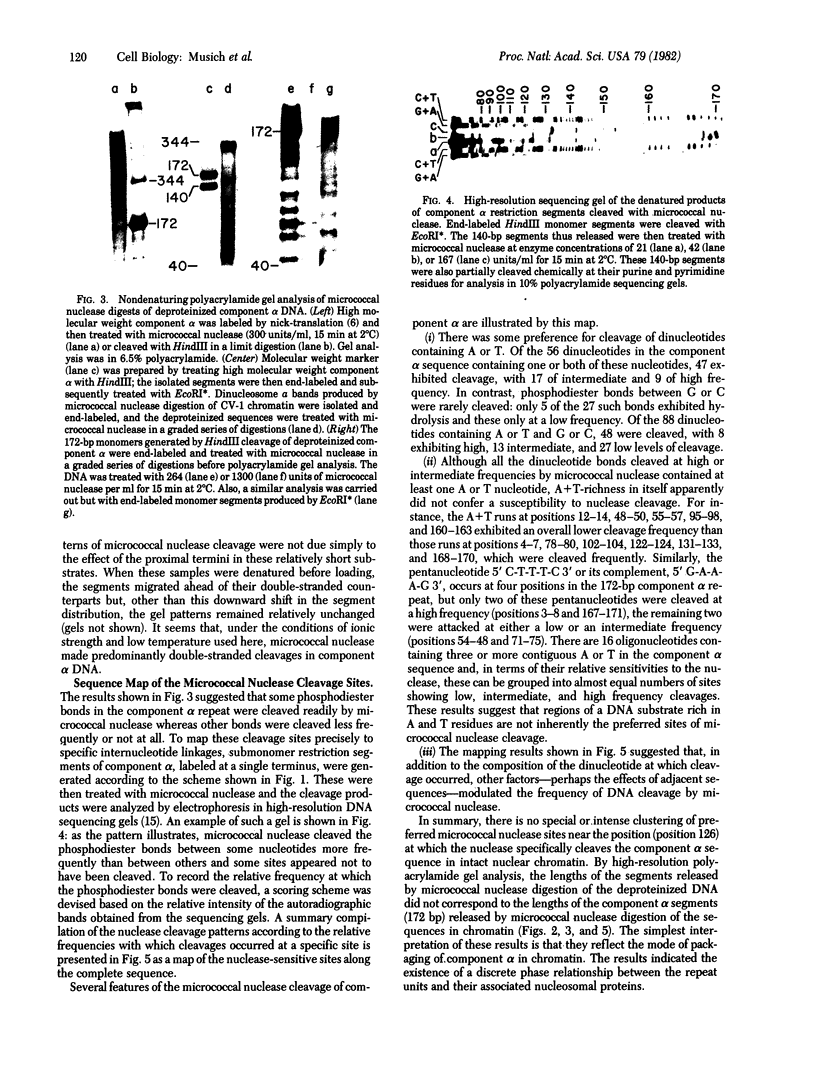
- Brown F. L., Musich P. R., Maio J. J. The repetitive sequence structure of component alpha DNA and its relationship to the nucleosomes of the African green monkey. J Mol Biol. 1979 Jul 15;131(4):777–799. doi: 10.1016/0022-2836(79)90201-8. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Lomonossoff G. P., Laskey R. A. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2659–2673. doi: 10.1093/nar/9.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittler F., Zachau H. G. Subunit structure of alpha-satellite DNA containing chromatin from African green monkey cells. Nucleic Acids Res. 1979 Sep 11;7(1):1–13. doi: 10.1093/nar/7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2643–2658. doi: 10.1093/nar/9.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C., Schedl P., Samal B., Worcel A. Chromatin structure of the 5S RNA genes of D. melanogaster. Cell. 1980 Nov;22(2 Pt 2):387–392. doi: 10.1016/0092-8674(80)90349-9. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., McKenna W. G., Musich P. R. Toward a molecular paleontology of primate genomes. II. The KpnI families of alphoid DNAs. Chromosoma. 1981;83(1):127–144. doi: 10.1007/BF00286020. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Toward a molecular paleontology of primate genomes. I. The HindIII and EcoRI dimer families of alphoid DNAs. Chromosoma. 1981;83(1):103–125. doi: 10.1007/BF00286019. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
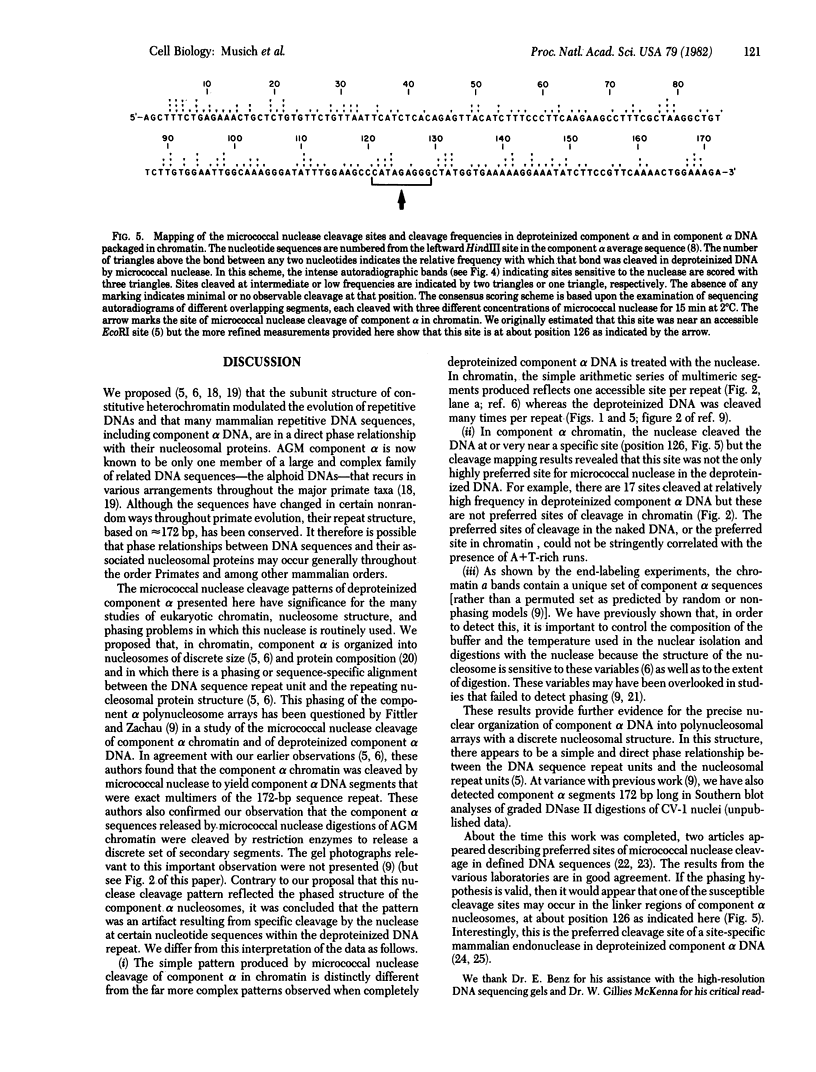
- McKenna W. G., Maio J. J., Brown F. L. Purification and properties of a mammalian endonuclease showing site-specific cleavage of DNA. J Biol Chem. 1981 Jun 25;256(12):6435–6443. [PubMed] [Google Scholar]
- Musich P. R., Brown F. L., Maio J. J. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: nucleosomal proteins associated with a highly repetitive mammalian DNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3297–3301. doi: 10.1073/pnas.74.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich P. R., Maio J. J., Brown F. L. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: indications of a phase relation between restriction sites and chromatin subunits in African green monkey and calf nuclei. J Mol Biol. 1977 Dec 15;117(3):657–677. doi: 10.1016/0022-2836(77)90063-8. [DOI] [PubMed] [Google Scholar]
- RUSHIZKY G. W., KNIGHT C. A., ROBERTS W. K., DEKKER C. A. Studies on the mechanism of action of micrococcal nuclease. II. Degradation of ribonucleic acid from tobacco mosaic virus. Biochim Biophys Acta. 1962 May 14;55:674–682. doi: 10.1016/0006-3002(62)90845-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Singer M., Rosenberg M. Highly reiterated sequences of SIMIANSIMIANSIMIANSIMIANSIMIAN. Science. 1978 Apr 28;200(4340):394–402. doi: 10.1126/science.205944. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Singer D. S. Arrangement of a highly repeated DNA sequence in the genome and chromatin of the African green monkey. J Biol Chem. 1979 Jun 25;254(12):5506–5514. [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Staphylococcal nuclease makes a single non-random cut in the simian virus 40 viral minichromosome. J Mol Biol. 1979 Aug 15;132(3):535–546. doi: 10.1016/0022-2836(79)90274-2. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wittig B., Wittig S. A phase relationship associates tRNA structural gene sequences with nucleosome cores. Cell. 1979 Dec;18(4):1173–1183. doi: 10.1016/0092-8674(79)90230-7. [DOI] [PubMed] [Google Scholar]