Abstract
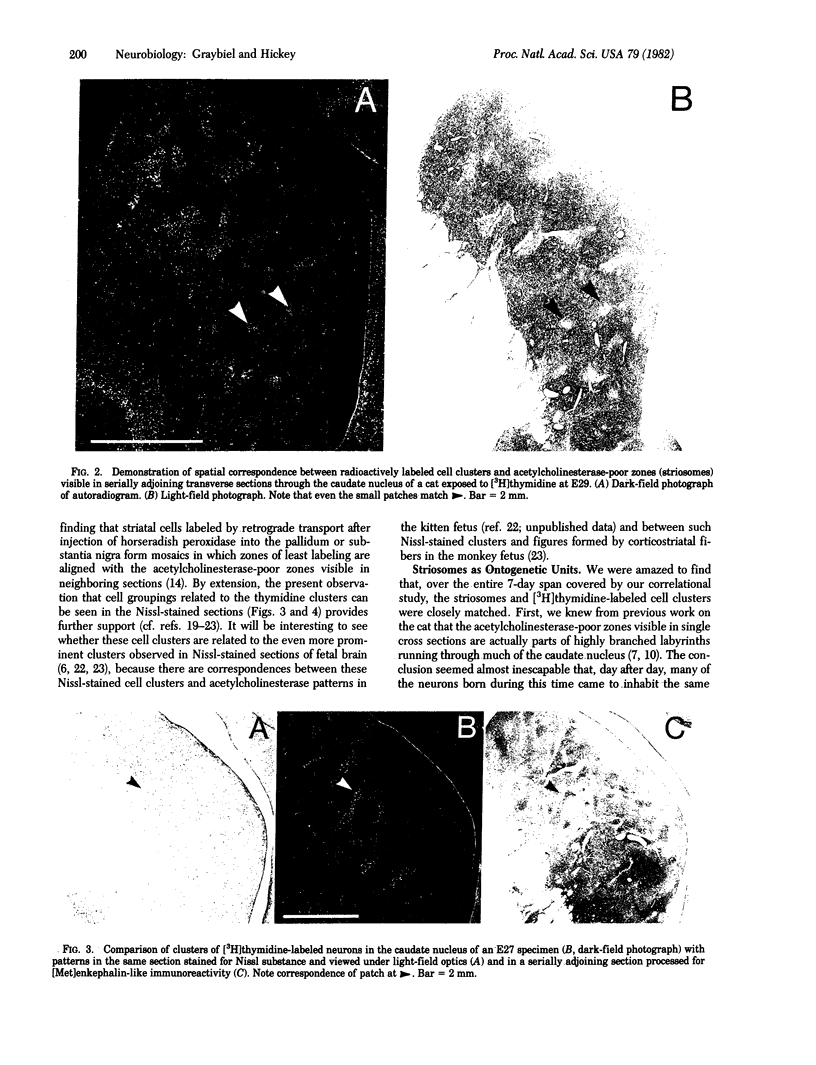
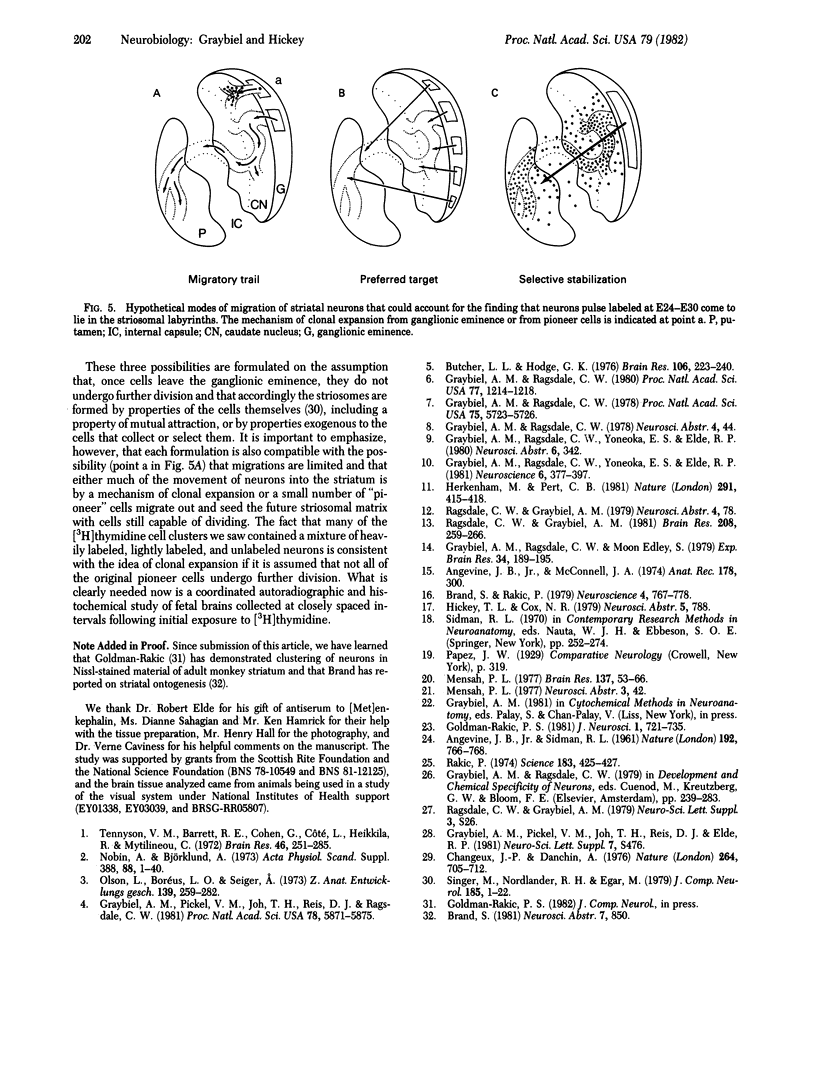
Neurons being generated in the striatum of 10 fetal cats were pulse labeled by injection of [3H]thymidine directly into the maternal uterus at times ranging between the 22nd and 30th days (E22-E30) of the 65-day gestational period. Many of the striatal neurons labeled during this interval were found, at adolescence, to form 100- to 600-mum-wide cell clusters in the caudate nucleus. In E24-E30 specimens, we compared the distributions of these cell clusters with the locations of patches of low acetylcholinesterase activity and high enkephalin immunoreactivity (the "striosomes') visualized in serially adjoining sections. We found precise matches between most of the cell clusters and the acetylcholinesterase-poor enkephalin-rich zones, regardless of the embryonic age at which exposure to the [3H]thymidine had occurred. We conclude that the histochemically distinct striosomal patch-works observed in the acetylcholinesterase and enkephalin preparations correspond to ontogenetic units of the striatum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angevine J. B., Jr, Sidman R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961 Nov 25;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Brand S., Rakic P. Genesis of the primate neostriatum: [3H]thymidine autoradiographic analysis of the time of neuron origin in the rhesus monkey. Neuroscience. 1979;4(6):767–778. doi: 10.1016/0306-4522(79)90005-8. [DOI] [PubMed] [Google Scholar]
- Butcher L. L., Hodge G. K. Postnatal development of acetylcholinesterase in the caudate-putamen nucleus and substantia nigra of rats. Brain Res. 1976 Apr 23;106(2):223–240. doi: 10.1016/0006-8993(76)91022-2. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. S. Prenatal formation of cortical input and development of cytoarchitectonic compartments in the neostriatum of the rhesus monkey. J Neurosci. 1981 Jul;1(7):721–735. doi: 10.1523/JNEUROSCI.01-07-00721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Pickel V. M., Joh T. H., Reis D. J., Ragsdale C. W., Jr Direct demonstration of a correspondence between the dopamine islands and acetylcholinesterase patches in the developing striatum. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5871–5875. doi: 10.1073/pnas.78.9.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Clumping of acetylcholinesterase activity in the developing striatum of the human fetus and young infant. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1214–1218. doi: 10.1073/pnas.77.2.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr, Moon Edley S. Compartments in the striatum of the cat observed by retrograde cell labeling. Exp Brain Res. 1979 Jan 2;34(1):189–195. doi: 10.1007/BF00238352. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr, Yoneoka E. S., Elde R. P. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6(3):377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981 Jun 4;291(5814):415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Mensah P. L. The internal organization of the mouse caudate nucleus: evidence for cell clustering and regional variation. Brain Res. 1977 Nov 25;137(1):53–66. doi: 10.1016/0006-8993(77)91012-5. [DOI] [PubMed] [Google Scholar]
- Nobin A., Björklund A. Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl. 1973;388:1–40. [PubMed] [Google Scholar]
- Olson L., Boréus L. O., Seiger A. Histochemical demonstration and mapping of 5-hydroxytryptamine- and catecholamine-containing neuron systems in the human fetal brain. Z Anat Entwicklungsgesch. 1973 Apr 16;139(3):259–282. doi: 10.1007/BF00519968. [DOI] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Graybiel A. M. The fronto-striatal projection in the cat and monkey and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 1981 Mar 16;208(2):259–266. doi: 10.1016/0006-8993(81)90556-4. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Singer M., Nordlander R. H., Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. J Comp Neurol. 1979 May 1;185(1):1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Tennyson V. M., Barrett R. E., Cohen G., Côté L., Heikkila R., Mytilineou C. The developing neostriatum of the rabbit: correlation of fluorescence histochemistry, electron microscopy, endogenous dopamine levels, and ( 3 H)dopamine uptake. Brain Res. 1972 Nov 13;46:251–285. doi: 10.1016/0006-8993(72)90019-4. [DOI] [PubMed] [Google Scholar]






