Abstract
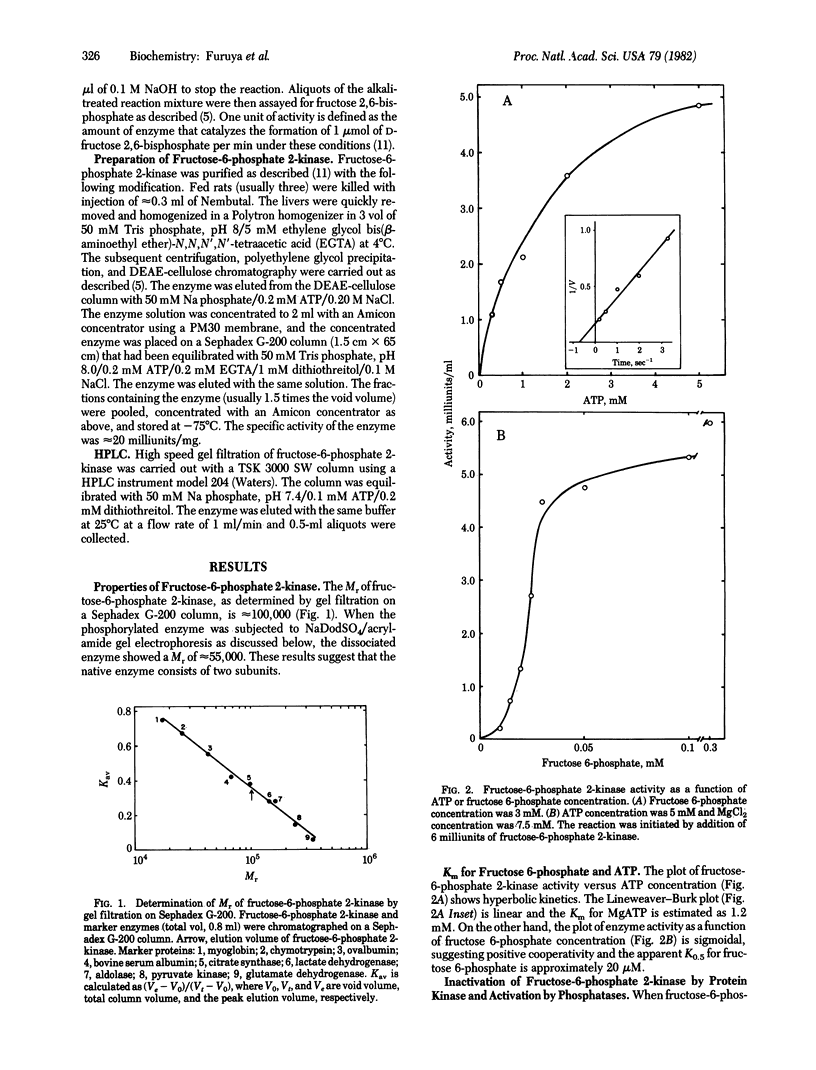
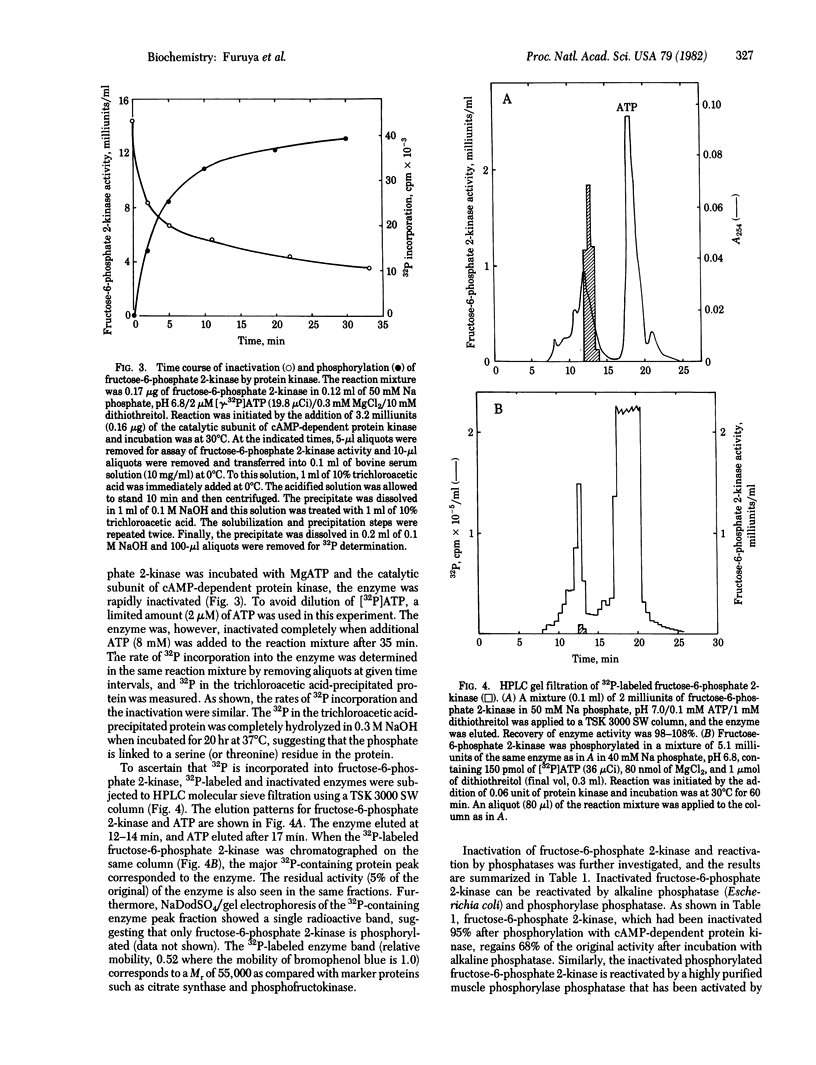
The kinetic properties and the control mechanism of fructose-6-phosphate 2-kinase (ATP: D-fructose-6-phosphate 2-phosphotransferase) were investigated. The molecular weight of the enzyme is approximately 100,000 as determined by gel filtration. The plot of initial velocity versus ATP concentration is hyperbolic with a Km of 1.2 mM. However, the plot of enzyme activity as a function of fructose-6-phosphate is sigmoidal. The apparent K0.5 for fructose-6-phosphate is 20 microM. Fructose-6-phosphate 2-kinase is inactivated by the catalytic subunit of cyclic AMP-dependent protein kinase, and the inactivation is closely correlated with phosphorylation. The enzyme is also inactivated by phosphorylase kinase in the presence of Ca2+ and calmodulin. The phosphorylated fructose-6-phosphate 2-kinase, which is inactive, is activated by phosphorylase phosphatase and alkaline phosphatase. The possible physiological significance of these observations in the coordinated control of glycogen metabolism and glycolysis is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Sul H. S., Walsh D. A. Phosphorylation and activation of the cardiac isoenzyme of phosphorylase kinase by the cAMP-dependent protein kinase. J Biol Chem. 1981 Aug 10;256(15):8030–8038. [PubMed] [Google Scholar]
- Furuya E., Uyeda K. A novel enzyme catalyzes the synthesis of activation factor from ATP and D-fructose-6-P. J Biol Chem. 1981 Jul 25;256(14):7109–7112. [PubMed] [Google Scholar]
- Furuya E., Uyeda K. An activation factor of liver phosphofructokinase. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5861–5864. doi: 10.1073/pnas.77.10.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya E., Uyeda K. Regulation of phosphofructokinase by a new mechanism. An activation factor binding to phosphorylated enzyme. J Biol Chem. 1980 Dec 25;255(24):11656–11659. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Pilkis S. J., El-Maghrabi M. R., Pilkis J., Claus T. H., Cumming D. A. Fructose 2,6-bisphosphate. A new activator of phosphofructokinase. J Biol Chem. 1981 Apr 10;256(7):3171–3174. [PubMed] [Google Scholar]
- Richards C. S., Furuya E., Uyeda K. Regulation of fructose 2,6-P2 concentration in isolated hepatocytes. Biochem Biophys Res Commun. 1981 Jun;100(4):1673–1679. doi: 10.1016/0006-291x(81)90711-7. [DOI] [PubMed] [Google Scholar]
- Richards C. S., Uyeda K. Changes in the concentration of activation factor for phosphofructokinase in hepatocytes in response to glucose and glucagon. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1535–1540. doi: 10.1016/s0006-291x(80)80040-4. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Aitken A., Bilham T., Condon G. D., Embi N., Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):529–537. [PubMed] [Google Scholar]
- Uyeda K., Furuya E., Luby L. J. The effect of natural and synthetic D-fructose 2,6-bisphosphate on the regulatory kinetic properties of liver and muscle phosphofructokinases. J Biol Chem. 1981 Aug 25;256(16):8394–8399. [PubMed] [Google Scholar]
- Uyeda K., Furuya E., Sherry A. D. The structure of "activation factor" for phosphofructokinase. J Biol Chem. 1981 Aug 25;256(16):8679–8684. [PubMed] [Google Scholar]
- Uyeda K. Phosphofructokinase. Adv Enzymol Relat Areas Mol Biol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- Uyeda K. Reaction of phosphofructokinase with maleic anhydride, succinic anhydride, and pyridoxal 5'-phosphate. Biochemistry. 1969 Jun;8(6):2366–2373. doi: 10.1021/bi00834a017. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Synthesis of a stimulator of phosphofructokinase, most likely fructose 2,6-bisphosphate, from phosphoric acid and fructose 6-phosphoric acid. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1524–1531. doi: 10.1016/0006-291x(80)91347-9. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):887–895. doi: 10.1042/bj1920887. [DOI] [PMC free article] [PubMed] [Google Scholar]


