Abstract
The Hfq protein mediates gene regulation by small RNAs (sRNAs) in about 50% of all bacteria. Depending on the species, phenotypic defects of an hfq mutant range from mild to severe. Here, we document that the purified Hfq protein of the plant pathogen and natural genetic engineer Agrobacterium tumefaciens binds to the previously described sRNA AbcR1 and its target mRNA atu2422, which codes for the substrate binding protein of an ABC transporter taking up proline and γ-aminobutyric acid (GABA). Several other ABC transporter components were overproduced in an hfq mutant compared to their levels in the parental strain, suggesting that Hfq plays a major role in controlling the uptake systems and metabolic versatility of A. tumefaciens. The hfq mutant showed delayed growth, altered cell morphology, and reduced motility. Although the DNA-transferring type IV secretion system was produced, tumor formation by the mutant strain was attenuated, demonstrating an important contribution of Hfq to plant transformation by A. tumefaciens.
INTRODUCTION
Agrobacterium tumefaciens is a broad-host-range phytopathogen that initiates the formation of tumors, called crown galls, on most dicotyledonous and some monocotyledonous plants (15). It is able to directly transform plant cells by transfer of the so-called T-DNA, which is part of a large tumor-inducing (Ti) plasmid (70). This transformation results in the overproduction of phytohormones responsible for tumor growth and the synthesis of opines metabolized by Agrobacterium (75). Interaction between A. tumefaciens and plant cells comes about in several steps (reviewed in reference 44). Briefly, wounded plant tissues release specific signals that induce the expression of Ti-plasmid-encoded virulence (vir) genes in A. tumefaciens. Following attachment to the plant cell, the bacterial type IV secretion system (T4SS) is established by diverse Vir proteins to export the T-DNA strand into the host. Once inside the plant cell, the T-DNA is delivered to the nucleus and integrated into the plant chromosome. After the expression of T-DNA genes, plant cells are reprogrammed for tumor growth and opine production.
The rhizosphere, the natural habitat of A. tumefaciens, is a densely populated, highly dynamic and competitive niche (17). To survive in such a harsh environment and to successfully infect plant cells, Agrobacterium has to monitor its surroundings and adapt its gene expression accordingly. We have previously found that small RNAs (sRNAs) play an important role in A. tumefaciens (68). Thus, we wondered whether the general RNA chaperone Hfq might be involved in controlling cellular processes and virulence in the phytopathogen.
The bacterial Sm-like protein Hfq is a critical component in the posttranscriptional control of gene expression (8, 63). Hfq is a largely conserved protein among bacteria, which was originally identified in Escherichia coli as a host factor essential for Qβ RNA bacteriophage replication (22). The protein assembles into ring-shaped homohexamers (38, 49).
During the last decade, Hfq has been recognized as a key factor in sRNA-mediated regulation in various enterobacteria (63, 66). In fact, the vast majority of all sRNAs characterized to date interact with this RNA chaperone (27). By preferentially binding to AU-rich single-stranded regions, Hfq was shown to facilitate short and imperfect base pairing between sRNAs and their mRNA targets (8, 27, 64). Often, interaction with the Shine-Dalgarno (SD) or anti-SD region renders the ribosome binding site (RBS) either more or less accessible and thereby modulates translation initiation. One example is the sRNA DsrA, which stimulates the expression of the RpoS stress response factor in Escherichia coli by binding to the 5′ leader of the rpoS mRNA (39, 46, 57). Hfq increases complex formation between DsrA and rpoS about 50-fold (57). In many cases, Hfq protects sRNAs from degradation by cellular RNases (reviewed in references 1, 8, and 63). Despite many sRNA-dependent functions, Hfq can also act alone as a translational repressor and can modulate mRNA decay by stimulating polyadenylation (20, 21). Additionally, roles of Hfq in tRNA biogenesis have been described (37).
Reflecting the broad variety of Hfq functions, mutations in the hfq gene were early observed to have a severe impact on bacterial physiology in E. coli, including alterations in growth rate, cell morphology, and tolerance to diverse stresses (62). Later on, phenotypic studies in numerous pathogenic organisms revealed dramatic virulence-related defects, from deficiencies in motility or host invasion to survival in the intracellular niche (reviewed in reference 11). Although Hfq has been most studied in gammaproteobacteria, there is increasing evidence that it is relevant in the alpha subgroup as well. For instance, in Brucella abortus, the hfq gene contributes to pathogenesis in mice (47). It was recently shown that Hfq coordinates the expression of the virulence-related type IV secretion system in this mammalian pathogen (10). In the photosynthetic alphaproteobacterium Rhodobacter sphaeroides, the Hfq protein plays an important role in protection against photooxidative stress (5, 6). During the past 2 years, several studies on Hfq in the plant symbiont Sinorhizobium meliloti demonstrated the importance of the RNA chaperone in cell physiology and symbiosis (3, 4, 24, 54, 60, 65).
The presence of more than 200 different sRNAs in A. tumefaciens (68) suggested that Hfq might be needed for sRNA-mediated gene regulation in this ubiquitous plant pathogen. Here, we demonstrate the influence of Hfq on the previously reported sRNA AbcR1 and its corresponding mRNA target atu2422 (69). We find at least eight proteins to be overproduced in an hfq mutant, which is characterized by altered cell morphology and reduced growth and motility, as well as significantly attenuated virulence, suggesting an important contribution of Hfq-mediated sRNA regulation to the Agrobacterium lifestyle.
MATERIALS AND METHODS
Bacterial growth conditions.
The bacterial strains and antibiotics used in this study are listed in Table S1 in the supplemental material. A. tumefaciens strains were cultivated in YEB complex medium or AB minimal medium (48a) with 1% glucose at 30°C. E. coli was grown in LB medium at 37°C.
Δhfq mutant construction.
The Δhfq mutant strain was constructed as described in Paulick et al. (43) with minor modifications. Upstream and downstream fragments (400 nucleotides) of the desired hfq gene region were amplified by PCR using the corresponding primer pairs (see Table S2 in the supplemental material). The fragments were ligated into the suicide vector pK19mobsacB. The resulting plasmid was introduced into A. tumefaciens by electroporation. Single-crossover integration mutants were selected on LB plates containing kanamycin. Single colonies were grown overnight in liquid LB without antibiotics and plated on LB containing 10% (wt/vol) sucrose to select for plasmid excision by double-crossover events. Kanamycin-sensitive colonies were checked for the targeted deletion by colony PCR and Northern blotting (see Fig. S2 in the supplemental material).
RNA preparation and Northern analysis.
Cells (10 ml) were harvested by centrifugation. After washing in ice-cold AE buffer (20 mM Na acetate, pH 5.5, 1 mM EDTA), pellets were immediately frozen in liquid nitrogen. Total RNA of cultured bacteria was isolated using the hot acid phenol method (2). To measure AbcR1 stability, rifampin was added to the cell cultures at a final concentration of 250 μg/ml and samples for RNA isolation were collected before (0 min) and 1, 5, and 10 min after the addition of the transcriptional inhibitor. Northern analyses were performed as previously described (69). Half-lives (t1/2) were estimated using AlphaEaseFC software (version 4.0.0; Alpha Innotec). The primers used for RNA or DNA probe generation are listed in Table S2 in the supplemental material.
Purification of Hfq and gel shift analyses.
Hfq His tag fusion and overproduction was carried out in E. coli BL21 cells, using the pET plasmid system (Novagen, Madison, WI). The protein was purified as previously described (45). The sRNA AbcR1 and the target atu2422 RNA fragment (comprising 50 nucleotides on either side of the ATG start codon [69]) were synthesized in vitro by runoff transcription with T7 RNA polymerase from the linearized plasmids runoff_C2A and runoff_atu2422, respectively. 5′-End labeling of AbcR1 was performed as described previously (7). Band shift experiments were performed in 1× structure buffer (Ambion, Austin, TX) in a total reaction mixture volume of 10 μl as follows. 5′-End-labeled AbcR1 sRNA (RNA corresponding to 5,000 cpm) and 1 μg of tRNA were incubated in the presence of increasing concentrations of purified Hfq protein (indicated in Fig. 1A) or in the presence of 1,000-fold excess Hfq and increasing concentrations of atu2422 RNA (indicated in Fig. 1B) at 30°C for 20 min. Prior to gel loading, the binding reaction mixtures were mixed with 3 μl of native loading dye (50% glycerol, 0.5× Tris-borate-EDTA [TBE], 0.1% bromophenol blue, and 0.1% xylenxyanol) and run on native 6% polyacrylamide gels in 0.5× TBE buffer at 250 V for 3 h.
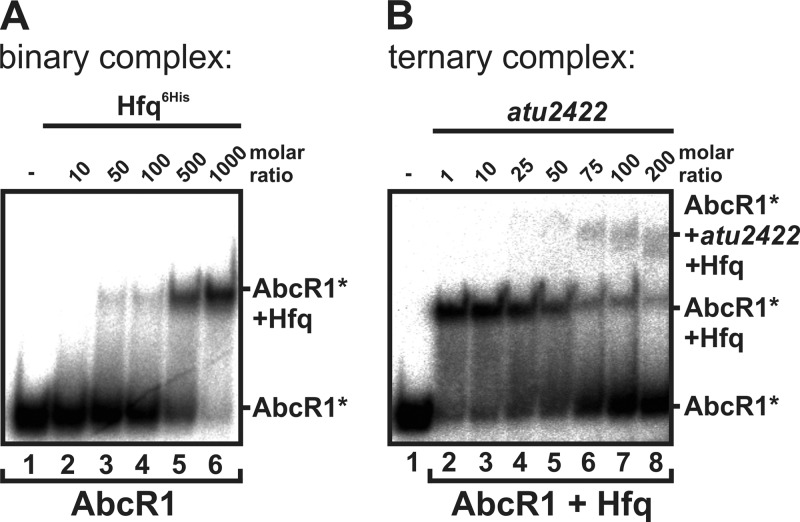
Fig 1.
Complex formation between Hfq, the sRNA AbcR1, and its target atu2422. (A) Gel retardation experiments with purified Hfq protein and AbcR1. 32P-labeled AbcR1 sRNA (0.07 pmol) was incubated with increasing concentrations of Hfq protein at 30°C for 20 min. Final concentrations (fold excess) of added Hfq are shown above the gel. Lane 1 contains a water control. (B) Gel retardation experiments with AbcR1, Hfq, and the target atu2422 mRNA fragment (100 nucleotides). 32P-labeled AbcR1 sRNA (0.07 pmol) was incubated with purified Hfq (1,000-fold excess) and increasing concentrations of atu2422. Final concentrations (fold excess) of added atu2422 RNA are shown above the gel. Lane 1 contains a water control. Samples were run on a 6% native gel at 250 V. The asterisks indicate radioactive (32P) labeling of the sRNA AbcR1.
Protein identification.
Proteins were separated by SDS-PAGE as described previously (48). Proteins from excised gel bands were subjected to in-gel digestion and analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) as described by Klüsener et al. (34).
Motility test.
Motility screening was carried out by spotting 5 μl of liquid overnight cultures on plates which contained AB minimal medium with an agar concentration of 0.5% (wt/vol), following incubation for 48 h at room temperature. For resistant strains, kanamycin was added to the soft agar plates.
Light microscopy.
Cells were grown in YEB medium to optical densities at 600 nm (OD600) of 0.15 and 0.5. For cell fixation, 10 μl of culture was mixed with 10 μl of 40°C prewarmed low-melting-point agarose on glass slides. After 2 h, images were taken with a light microscope (BX51; Olympus, Essex, United Kingdom).
Plant infection experiments.
Overnight cultures of A. tumefaciens wild-type (WT) and Δhfq cultures were inoculated to an OD600 of 0.1 in YEB medium and grown to an OD600 above 0.2. Cultures were diluted to final OD600 values of 0.2 and 0.02 in YEB medium. Leaves of Kalanchoe daigremontiana were wounded with a sterile scalpel, and 10-μl amounts of the bacterial suspensions were applied to the wounds. Plants were incubated at room temperature for 4 to 6 weeks until tumor formation was detected.
Quantitative tumorigenesis assays with potato tuber discs were carried out as described before (61). Bacterial cells at OD600 values of 0.7 to 1.0 were collected and resuspended in phosphate-buffered saline at 108 and 106 cells/ml for inoculation. The potato tuber discs were placed on water agar, infected with 10 μl of bacterial culture, and incubated at 23°C for 2 days. The discs were then placed on water agar supplemented with 100 μg/ml ticarcillin, to kill bacteria, and incubated at 23°C (16 h light and 8 h darkness) for 3 weeks before tumors were scored. Western blot analysis to check VirB protein expression was performed as previously described (67).
RESULTS
AbcR1 is an Hfq target.
We previously described the A. tumefaciens sRNA AbcR1, which controls the translation and stability of the atu2422 mRNA, coding for a proline and γ-aminobutyric acid (GABA) transporter (13, 69). The interaction regions of AbcR1 and atu2422 were mapped to an accessible loop in the sRNA and the SD region in the mRNA (69). AbcR1 is encoded in tandem with a similar sRNA, called AbcR2. Owing to differences in the loop sequences, it cannot functionally replace AbcR1 and its target mRNAs are presently unknown. Here, we examined whether Hfq plays a role in the interaction between AbcR1 and atu2422. To this end, we produced recombinant Hfq and performed gel retardation experiments according to established procedures (21, 40, 53). The A. tumefaciens Hfq protein fused to a His tag was produced in E. coli BL21 cells and purified by Ni-nitrilotriacetic acid chromatography (see Fig. S1 in the supplemental material). 32P-labeled AbcR1 sRNA was incubated with increasing concentrations of Hfq protein prior to separation on a native polyacrylamide gel. The addition of Hfq resulted in complex formation with AbcR1 (Fig. 1A).
Hfq proteins are generally thought to facilitate sRNA-mRNA interactions by simultaneous binding of both RNA molecules (31, 56, 64). To test this hypothesis, increasing concentrations of the target mRNA fragment atu2422 were added to radioactively labeled AbcR1 sRNA preincubated with Hfq. RNA fragments were chosen based on the previously mapped interaction sites of AbcR1 and atu2422 (69). The binary Hfq-AbcR1 complex (Fig. 1A) was converted into a ternary complex after the addition of the target mRNA (Fig. 1B). The formation of this complex was dependent on the specific interaction between AbcR1 and atu2422, as AbcR1 and a nontarget RNA did not promote the formation of a ternary complex (data not shown). In line with the model that RNAs cycle on Hfq (19), high concentrations of the atu2422 RNA displaced AbcR1 (Fig. 1B).
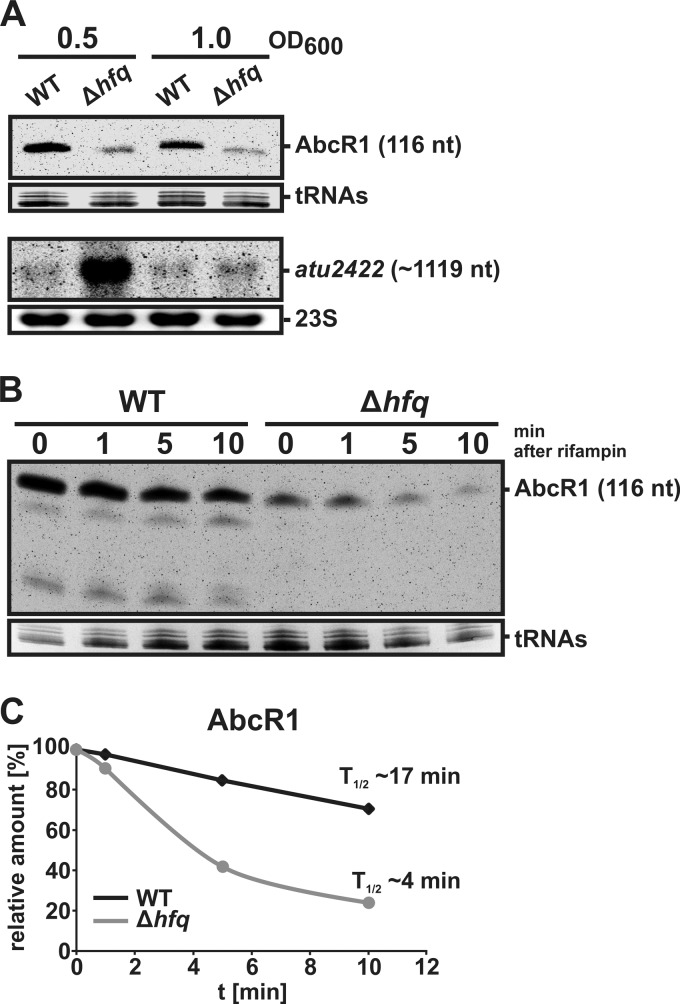
To test whether Hfq influences AbcR1 in vivo, we constructed an in-frame deletion strain of hfq. The absence of hfq in the mutant was confirmed by colony PCR and Northern blot analysis, which also shows that hfq forms a monocistronic transcript in the WT strain (see Fig. S2 in the supplemental material). In vivo evidence for the involvement of Hfq in the interaction of AbcR1 and atu2422 was provided by Northern blot analysis (Fig. 2A). AbcR1 and its target atu2422 were detected by specific RNA probes in different growth phases. Compared to the WT levels, the AbcR1 signal was strongly reduced in the hfq deletion strain under both conditions. Fully consistent with our previous observation that AbcR1 decreases the level and stability of atu2422 mRNA (69), smaller amounts of the sRNA AbcR1 in the hfq deletion mutant correlated with elevated amounts of atu2422 mRNA, particularly in the exponential growth phase.
Fig 2.
Effect of Hfq on AbcR1-mediated gene regulation in vivo. (A) Northern blot analysis of AbcR1 and atu2422 in A. tumefaciens wild type (WT) and the Δhfq strain. Hybridizations were performed with 8 μg of total RNA from cells grown in YEB complex medium to the optical densities indicated above the gels. Primers used for RNA probe generation are listed in Table S2 in the supplemental material. Ethidium bromide-stained 23S RNAs or tRNAs were used as loading controls. nt, nucleotides. (B) Stability of AbcR1. WT and Δhfq mutant cells were grown to an OD600 of 1.0 in YEB medium. Samples were taken before (0) and 1, 5, and 10 min after the addition of rifampin (250 μg/ml). Amounts of 8 μg of total RNA were separated on a 10% polyacrylamide gel containing 7 M urea and were detected by Northern analysis using a DNA probe (generated by primers DNAprobe_C2A_fw and DNAprobe_C2A_rv). The size of AbcR1 is given on the right. Ethidium bromide-stained tRNAs were used as loading controls. (C) Relative amounts of AbcR1 in A. tumefaciens WT and the hfq mutant after the addition of rifampin. Northern blot signals (B) were quantified using AlphaEaseFC software.
To assess the stability of AbcR1 in Agrobacterium WT and the hfq mutant, both strains were grown to mid-stationary phase (OD600 of 1) before transcription was stopped by the addition of rifampin. The half-life of AbcR1 was determined by Northern blot analysis (Fig. 2B). In the presence of Hfq, AbcR1 had an estimated half-life of about 17 min (Fig. 2C). In contrast, the sRNA was less abundant and decayed more quickly (t1/2 ∼ 4 min) in the hfq mutant. In summary, the in vitro and in vivo results consistently show an important role of A. tumefaciens Hfq in AbcR1-mediated regulation.
The absence of Hfq alters the expression of various genes in A. tumefaciens.
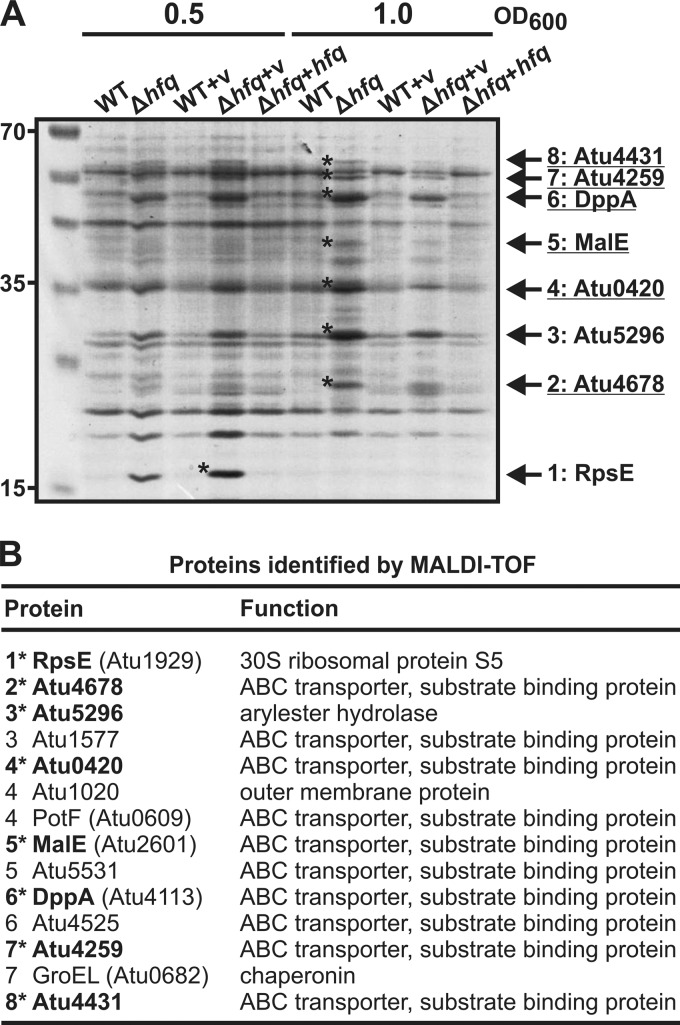
Next, we determined Hfq-dependent changes in protein production and compared the protein extracts from WT and Δhfq cells grown to exponential and mid-stationary phase in YEB complex medium by SDS-PAGE. As controls, a strain in which Δhfq was complemented by plasmid-encoded hfq and WT and Δhfq strains carrying the empty vector were used (Fig. 3A). The protein profiles of the WT and the hfq mutant differed significantly under both growth conditions. At least eight proteins were visibly overrepresented in the mutant strain compared to their levels in the WT. In all cases, the abundance of these proteins was restored to WT-like levels in the complemented Δhfq mutant, demonstrating that the observed changes were Hfq dependent.
Fig 3.
Altered protein levels in the hfq deletion mutant. (A) Total protein samples from different growth phases (indicated above the gels) of A. tumefaciens wild type (WT), the Δhfq strain, the complemented mutant (Δhfq+hfq), and control strains containing the empty vector (v) (WT+v and Δhfq+v) were loaded on a 12% SDS–PAGE gel. The positions of marker proteins are given on the left in kDa. Eight prominent protein bands accumulating in the mutant were analyzed via MALDI-TOF. The proteins identified with the highest confidence are given on the right. ABC transporter components are underlined. (B) Identification of overexpressed proteins (A) in the Δhfq mutant by MALDI-TOF. Proteins that were ranked at the first position after MALDI-TOF analysis are in boldface and marked by asterisks. All other listed proteins were additionally identified during mass spectrometry.
Proteins overrepresented in the hfq mutant were excised from the gel and subjected to mass spectrometry. Although some of the proteins could not be identified unambiguously (Fig. 3B), it was evident that the majority of these proteins were substrate-binding proteins of the ABC transporter family (underlined in Fig. 3A). In contrast to all other proteins, which accumulated in late stationary phase, the 20.5-kDa ribosomal protein RpsE was most abundant in Δhfq cells from early-exponential-phase cultures.
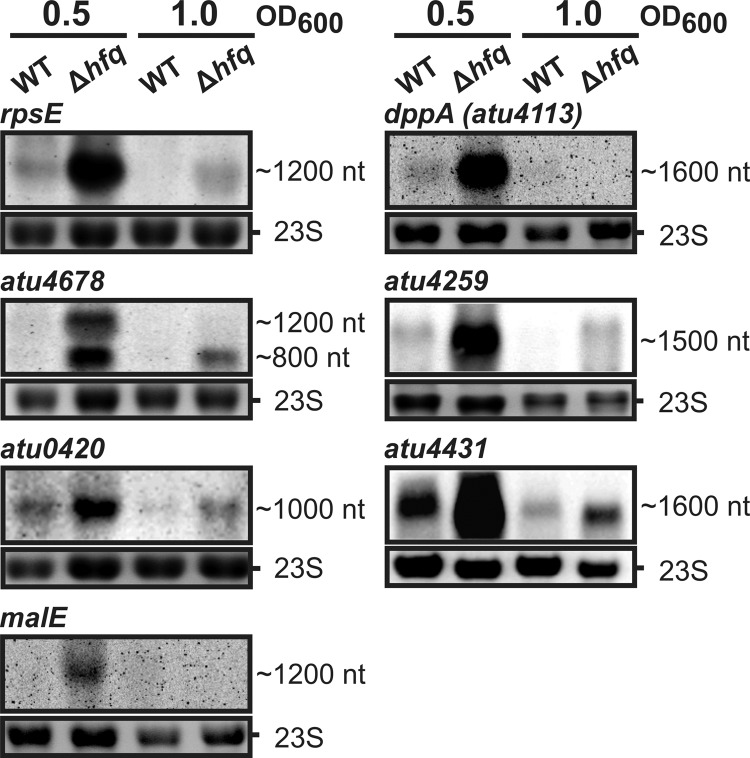
To analyze the RNA levels of the Hfq-affected proteins, we selected seven examples and performed Northern hybridizations with RNA isolated from cultures grown to the optical density used in the SDS-PAGE (Fig. 4). The corresponding mRNAs were overrepresented already in exponential growth phase in the Δhfq strain compared to the WT levels.
Fig 4.
Altered RNA levels in the hfq deletion mutant. Northern blots determining RNA levels of some of the proteins identified by SDS-PAGE in Fig. 3A. Hybridizations were performed with amounts of 8 μg of total RNA from A. tumefaciens wild type (WT) and the Δhfq mutant grown to the optical densities indicated above. Primers used for RNA probe generation are listed in Table S2 in the supplemental material. Ethidium bromide-stained 23S RNAs were used as loading controls.
Phenotypic defects of the hfq mutant.
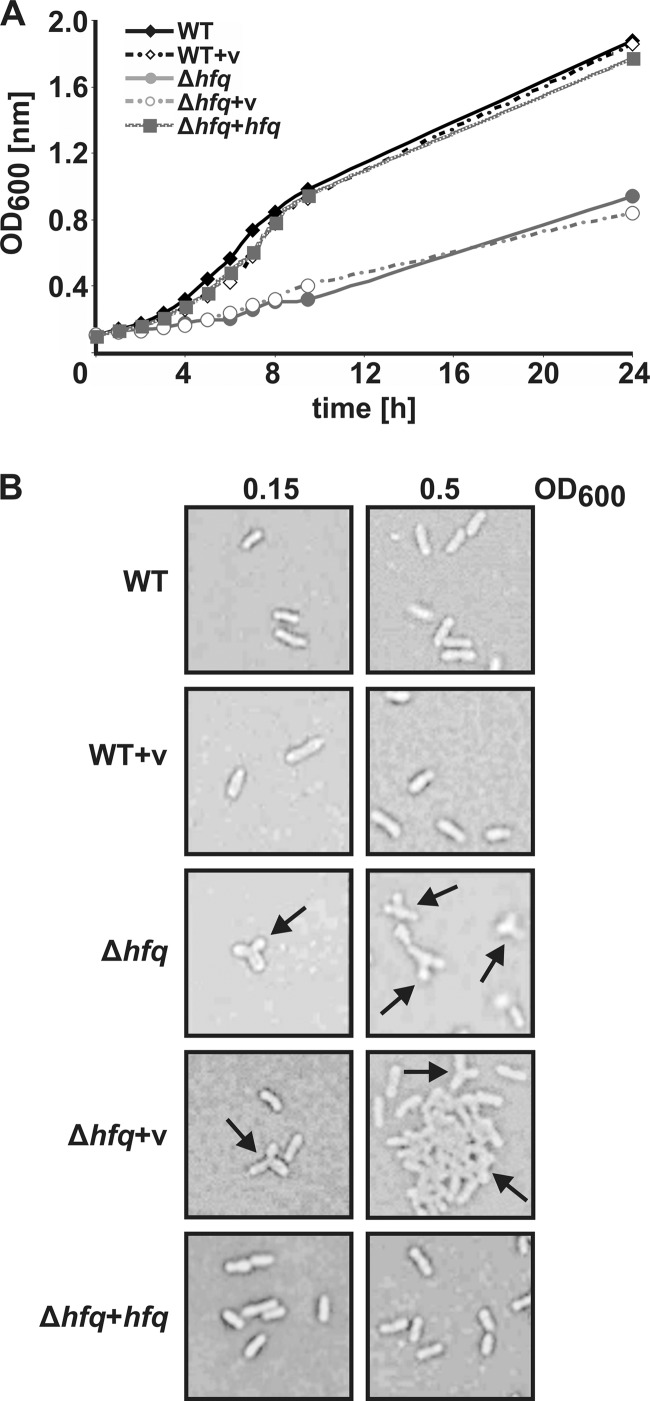
The observed alterations in the protein, mRNA, and sRNA concentrations in the hfq mutant in comparison to those in the parental strain (Fig. 1 to 4) suggested a contribution of Hfq to diverse cellular pathways in A. tumefaciens and led us to conduct a phenotypic characterization. Consistent with the delayed growth and formation of small colonies on YEB medium agar plates at 30°C (data not shown), the hfq mutant showed a longer lag phase after inoculation into fresh medium and reached lower optical densities than the WT (Fig. 5A). Furthermore, the mutant cultures started to form visible cell aggregates at an OD600 of around 0.2 (data not shown; for OD measurements, cells were resuspended by vortexing, and CFU were determined to support the OD reading results). The growth of the complemented Δhfq strain was indistinguishable from that of the WT.
Fig 5.
Growth defect and altered cell morphology of the Δhfq strain. (A) A. tumefaciens wild type (WT), the Δhfq mutant, the complemented mutant (Δhfq+hfq), and corresponding control strains harboring the empty vector (v) (WT+v and Δhfq+v) were grown in YEB complex medium, and OD600 values were plotted over time. Three independent experiments gave comparable results. (B) Microscopic images were taken of A. tumefaciens strains from cultures grown in YEB medium to optical densities indicated above the images. Y-shaped cells are indicated by arrows.
To analyze cell morphology, aliquots of liquid cultures grown in YEB medium to optical densities (OD600) of 0.15 and 0.5 were fixed on glass slides and inspected by light microscopy. Both the WT and the WT with the empty vector formed typical rod-shaped cells (Fig. 5B). In contrast, many Δhfq cells showed an unusual Y-shaped appearance. Counting of 1,800 cells from exponential growth culture and 2,000 cells from stationary-phase culture revealed that 8.7 and 7.8%, respectively, were Y shaped. Consistent with the flocking phenotype observed in liquid cultures, aggregated cells were visible in Δhfq cultures from exponential growth (OD600 of 0.5). This abnormal morphology was completely restored in strains expressing hfq from the complementation plasmid.
Next, we compared the motility of the WT and the Δhfq strains. WT cells were motile and formed concentric rings around the point of inoculation. In contrast, the motility of the hfq mutant was consistently reduced by 25%.
Hfq is a virulence determinant in A. tumefaciens.
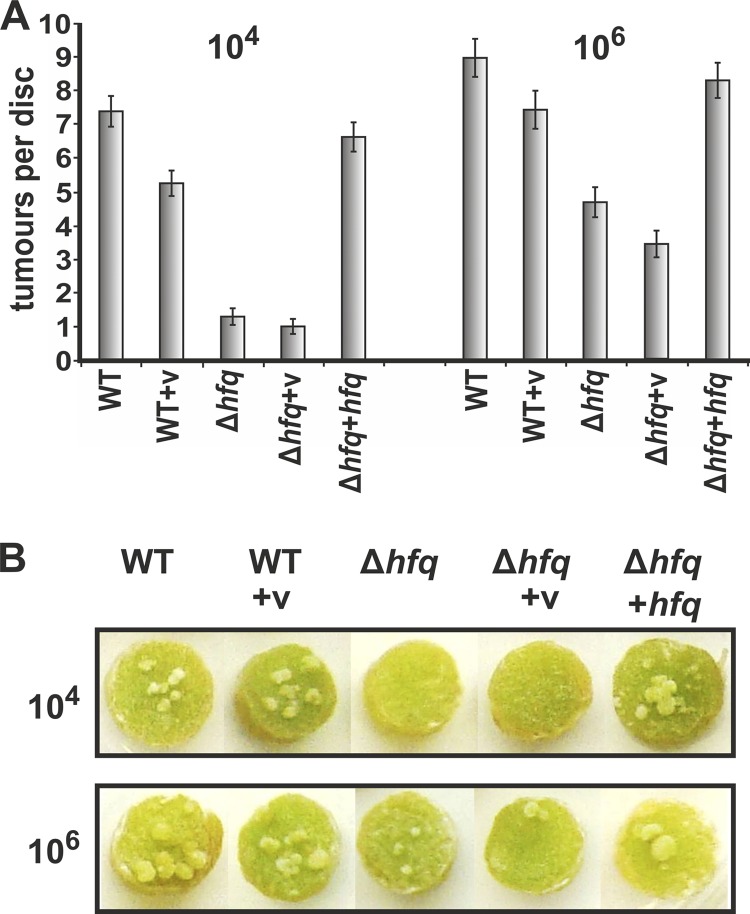
To determine if the absence of hfq influences the virulence of A. tumefaciens, we used two different tumor formation assays. First, cells were cultivated in YEB medium to low optical densities and used to transform living plants. Infection of freshly wounded Kalanchoe leaves with the A. tumefaciens WT and WT cells harboring the empty vector led to efficient tumor formation that was visible 4 weeks after inoculation (see Fig. S3 in the supplemental material). In contrast, leaves inoculated with the hfq mutant were severely delayed in tumor formation and produced hardly detectable tumors or no tumors at all. Complementation by ectopically expressed hfq restored tumor formation.
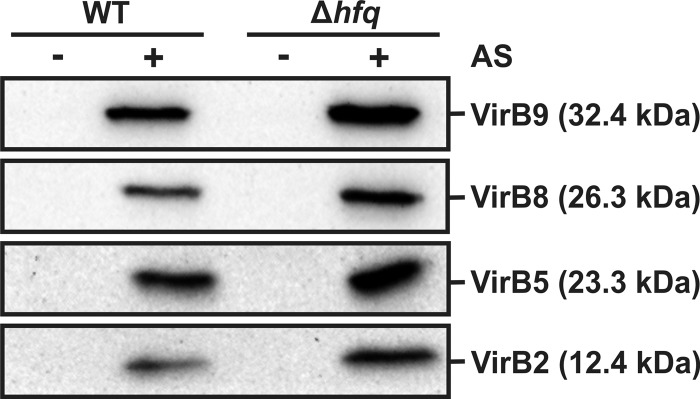
More-quantitative results were obtained on potato tuber discs (Fig. 6). A. tumefaciens WT (104 cells/disc) elicited approximately seven tumors per disc after 3 weeks of incubation. Potato discs infected with the Δhfq strain showed at most one tumor after the same incubation time. When 100-fold-higher cell densities were used for infection (106 cells/disc), the virulence defect was less severe. Four to five tumors were counted on Δhfq-infected potato discs, suggesting that deletion of hfq does not completely abolish the ability of A. tumefaciens to genetically manipulate plant cells. Interestingly, the hfq mutant still expresses the type IV secretion machinery, as four different VirB proteins were detected at WT levels by Western blot analysis (Fig. 7).
Fig 6.
Virulence defect of the A. tumefaciens Δhfq strain on potato discs. Quantitative tumor formation assay on potato tuber discs. (A) Amounts of 104 and 106 cells/disc of A. tumefaciens wild type (WT), the Δhfq mutant, the complemented mutant (Δhfq+hfq), and control strains harboring the empty vector (v) (WT+v and Δhfq+v) were examined for their tumor formation efficiency on potato discs. Tumorigenesis efficiency is scored by the number of tumors per disc (mean value calculated from results of 60 potato tuber discs for each strain in each independent experiment; error bars show standard errors of the means). (B) Representative pictures of tumors on potato discs. Two independent experiments gave similar results.
Fig 7.
Presence of T4SS proteins in the WT and hfq mutant. Cells were grown under non-virulence-inducing (− AS) or virulence-inducing conditions (+ AS [1 mM]) in AB minimal medium (AS, acetosyringone). Cell lysates were subjected to SDS-PAGE, followed by Western blotting and detection with specific antisera.
DISCUSSION
Hfq-dependent ABC transporter regulation.
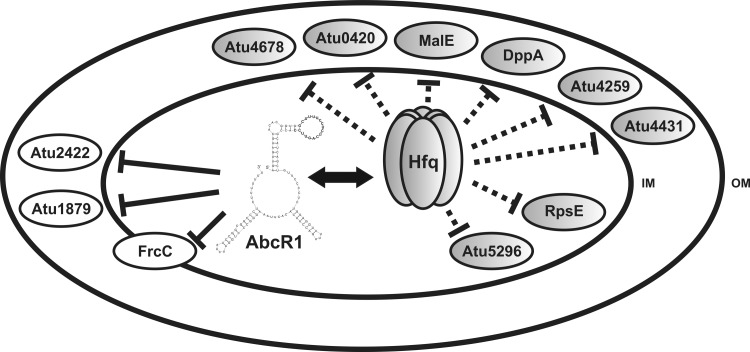
Soil bacteria like Rhizobium and Agrobacterium are highly adaptive heterotrophic organisms, able to assimilate a wide range of carbon and nitrogen sources in the rhizosphere. Their metabolic plasticity is reflected in their large genome sizes, with many genes devoted to transport and catabolic pathways. S. meliloti and A. tumefaciens both encode more than 150 ABC transporter systems (23, 70), which ought to be tightly regulated in response to nutrient availability. In the present study, the A. tumefaciens Hfq protein was shown to play a major role in the regulation of a number of periplasmic binding proteins of ABC transporters (Fig. 8). Six of eight significantly overrepresented proteins in the Δhfq mutant belonged to the ABC transporter family. The extensive role of Hfq in the regulation of nutrient uptake systems has also been demonstrated in global analyses of other hfq deletion mutants (25, 30, 50, 52, 53, 55). Recent studies in the plant symbiont S. meliloti revealed ABC transporter-related genes as the dominant class affected by hfq mutation (3, 24, 60). In accordance, Hfq-mediated regulation of ABC transporter uptake mechanisms seems to be a common theme among bacteria. Our phenotypic characterization suggests that Hfq-mediated gene regulation has many functions beyond balancing the nutrient supply.
Fig 8.
Current model of Hfq-dependent processes in A. tumefaciens. A schematic A. tumefaciens cell is depicted with inner and outer membrane (IM and OM, respectively). mRNAs influenced by Hfq mostly code for periplasmic proteins. Hfq-mediated regulation is exerted via the sRNA AbcR1 (69) (continuous lines) or via yet-to-be-determined sRNAs (dashed lines).
Hfq is largely known to function as a global player in sRNA regulatory pathways (reviewed in references 8 and 66). We recently reported more than 200 sRNA candidates in A. tumefaciens (68). Here, we demonstrated the well-characterized sRNA AbcR1 to be modulated by Hfq. AbcR1 regulates the expression of at least three ABC transporter-related mRNAs, namely, atu2422, coding for a GABA and proline binding protein, atu1879, coding for a periplasmic binding protein of unknown function, and frcC, coding for a putative sugar transporter (69). Amino acid transport is also regulated by AbcR1 and the related AbcR2 in Brucella abortus (9). The formation of a ternary complex between A. tumefaciens AbcR1, Hfq, and the target mRNA atu2422 in vitro and the Hfq-dependent regulation of AbcR1 and atu2422 RNA levels in vivo suggest a direct contribution of Hfq to AbcR1-mediated regulation. Furthermore, AbcR1 was stabilized by Hfq in vivo, like its S. meliloti homologue SmrC16 (65). AbcR1 stability was reduced 4-fold in the absence of Hfq. Two- to 6-fold-reduced half-lives of SmrC16 in an S. meliloti hfq mutant suggest a common mechanism of regulation for AbcR1 and SmrC16.
Analysis of protein amounts by SDS-PAGE revealed eight visibly overproduced proteins in the hfq mutant (Fig. 3A). Northern blot analysis further confirmed an increase of the mRNA levels of most of these proteins in the absence of Hfq (Fig. 4), suggesting that sRNA-dependent translational blockage might be coupled to mRNA degradation. Since none of the three known AbcR1 targets was among those proteins, we expect the list of sRNA-controlled transporter genes in A. tumefaciens to grow in the future. Global approaches to identify Hfq-interacting mRNAs and sRNAs (coimmunoprecipitation), as well as affected proteins (2-dimensional PAGE), are expected to provide further insights into the physiological role of Hfq in A. tumefaciens.
Phenotypes of the hfq mutant.
The hfq deletion strain generated during this study displayed alterations in growth, cell morphology, motility, and virulence. Although hfq mutants often exhibit a pleiotropic phenotype, the severity is species dependent (3, 14, 24, 51, 53, 60). Slower growth of the A. tumefaciens hfq mutant might be a consequence of the misregulation of ABC transporter genes, which is expected to result in altered nutrient acquisition, as has been shown for GABA uptake in the AbcR1 mutant (69).
A particularly interesting protein overproduced in the hfq mutant was RpsE (ribosomal protein S5), which has been implicated in tRNA selection and translation fidelity in E. coli (72). In contrast to the ABC transporter proteins, A. tumefaciens RpsE accumulated mostly in the earlier growth phase. Although it remains unclear how Hfq affects the RpsE level, the Northern hybridization showed that rpsE mRNA was dramatically induced in the absence of the RNA chaperone. Our study provides evidence that the unbalanced production of ribosomal proteins during exponential growth, probably associated with reduced translation accuracy, might be another reason for the pleiotropic defects of hfq mutants. Consistent with this notion, various mRNAs coding for ribosomal proteins coimmunoprecipitated with E. coli (73) or R. sphaeroides (6) Hfq.
Light microscopy revealed that about 8% of all Δhfq cells are atypically branched, exhibiting a Y shape. This phenotype has previously been correlated with blocked cell division in rhizobia (36). The Y-shaped morphology is reminiscent of A. tumefaciens cells lacking the Lon protease (58). This ATP-dependent protease plays a primary role in the degradation of misfolded proteins (26). In A. tumefaciens, it might also be involved in the control of lipopolysaccharide (LPS) biosynthesis (35). Defects in LPS production often lead to pleiotrophic phenotypes. Another substrate of Lon in alphaproteobacteria is CcrM, a DNA methyltransferase that plays a critical role in DNA replication and cell division in A. tumefaciens and other rhizobia (33). The majority of A. tumefaciens cells overexpressing CcrM show a branched appearance like that of the hfq mutant (33, 71). Notably, differential expression of the lon gene has been reported in E. coli (downregulation) and Yersinia pestis (upregulation) hfq mutants (25, 30). The question of which Hfq target(s) are responsible for the changes in cell morphology and growth defects remains to be addressed in many organisms.
The abnormal cell morphology and impaired growth might be responsible in part for the reduced motility of the A. tumefaciens Δhfq strain. Alternatively, the defect may be caused by an alteration in the expression of flagellum-associated genes. In the closely related organism S. meliloti, transcripts of most known flagellar, chemotaxis, and motility genes were decreased in the hfq mutant (24). Changes in flagellum proteins were also reported for Salmonella enterica serovar Typhimurium and E. coli (51, 53). However, protein gels of the A. tumefaciens hfq mutant (Fig. 3A) and Western blot analysis with flagellum-specific antisera (data not shown) did not reveal any obvious changes in the presence of flagellum proteins. In summary, our study revealed that important cellular processes, such as nutrient acquisition, the composition of the translation machinery, and cell division, are affected in the absence of Hfq in A. tumefaciens.
Hfq is required for efficient plant transformation.
Although virulence-related defects are not uncommon in bacterial hfq mutants (reviewed in reference 11), our study reports the first Hfq-associated virulence phenotype in a plant pathogen, emphasizing the universal importance of Hfq in host-microbe interactions. In response to plant-released signals, A. tumefaciens induces the vir regulon, encoding the components of the T4SS, which is required for T-DNA transport into the host (reviewed in reference 44). Unlike a B. abortus hfq mutant (10) and virulence-defective A. tumefaciens mutants with altered membrane composition (67), the Agrobacterium hfq mutant produced normal amounts of VirB proteins. This suggests that the signal transduction pathway, from perception of wounded plant tissue to expression of the T4SS, is not compromised by the hfq deletion. Transient infection assays with Arabidopsis thaliana root segments (32, 74), however, showed 2- to 3-fold-reduced T-DNA transfer by the hfq mutant (data not shown). These data imply a role of hfq at early step(s) of the Agrobacterium transformation process prior to T-DNA integration, because transient expression of T-DNA genes does not require the integration of T-DNA into the plant genome (41). The underlying mechanism remains to be established.
Since motility and chemotaxis toward plant-derived signals influence the infection process (12), the swimming defect of the A. tumefaciens Δhfq strain might contribute to the observed virulence phenotype. Motility generally is an important parameter in host cell colonization and was shown to be dependent on Hfq in E. coli, Pseudomonas aeruginosa, and S. Typhimurium (51, 53, 55). Nonetheless, we expect the effect of reduced motility on virulence to be of minor importance in our assays, because bacterial cells were directly applied onto wounded Kalanchoe leaves or potato discs. Even nonmotile A. tumefaciens strains were shown to retain 64% virulence on red potato tuber discs compared to the virulence of the parental WT strain (12).
The abnormal cell morphology of the A. tumefaciens hfq mutant and its tendency to form cell aggregates might be another reason for reduced plant transformation. Other A. tumefaciens mutants, displaying similar Y-shaped cell types, showed significant virulence defects, similar to those of the hfq mutant (16, 58). It is striking, however, that even 106 cells of the hfq mutant did not form as many tumors as 104 A. tumefaciens WT cells. Since more than 90% of Δhfq cells are normally shaped, it is unlikely that the Y-shaped cells play a dominant role in the virulence defect in such high bacterial concentrations.
Another attractive yet theoretical hypothesis is that A. tumefaciens transfers sRNAs along with its T-DNA into the host during plant transformation to subvert plant defense mechanisms or to modulate host metabolism for its own benefit. Interestingly, five putative sRNAs were shown to be expressed from the T-DNA region (68).
Interestingly, the type IV secretion system seemed to be intact in the A. tumefaciens hfq mutant. Although secretion of RNA into host cells as an effective infection strategy has been hypothesized (29, 42, 66), it has never been firmly proven. Given the universal importance of microRNAs in eukaryotic gene regulation, it is also tempting to speculate that the T-DNA might express such regulatory RNAs once it is integrated into the plant genome. Several recent findings suggest that this speculation might not be too far-fetched. A deep-sequencing approach revealed more than 20 miRNAs differentially expressed from soybean roots in response to inoculation with the symbiont Bradyrhizobium japonicum (59). Several DNA viruses, like the Kaposi's sarcoma-associated herpesvirus, express miRNAs in infected cells to downregulate cellular mRNAs (28). Most interestingly, the plant miRNA pathway is essential for Agrobacterium disease development. Plants mutated in RNA silencing pathways showed near immunity to A. tumefaciens infection (18). In silico predictions identified some putative miRNA precursors on the T-DNA sequence (data not shown). Although experimental validation of those regulatory RNAs is still pending, they indicate the regulatory potential of the T-DNA. In light of these reports, it seems possible that in addition to cumulative effects of several phenotypic alterations in the hfq mutant, Hfq-dependent sRNAs might play a more direct role in tumor formation by A. tumefaciens.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christian Baron (Montreal) for VirB-specific antisera and Knut Büttner (Greifswald) for MALDI-MS analysis. Members of the RNA group in the Narberhaus laboratory are acknowledged for helpful discussions. We also acknowledge the technical assistance of Yi-Chieh Wang for Arabidopsis root assays.
This work was supported by a grant from the German Research Foundation (DFG priority program SPP 1258: Sensory and Regulatory RNAs in Prokaryotes) to F.N., a fellowship from the RUB Research School to I.W., and a joint grant from the German Academic Exchange Service (DAAD) and the Taiwan National Science Council (100-2911-I-001-053) to F.N. and E.-M.L.
Footnotes
Published ahead of print 20 July 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Aiba H. 2007. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr. Opin. Microbiol. 10:134–139 [DOI] [PubMed] [Google Scholar]
- 2. Aiba H, Adhya S, de Crombrugghe B. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 256:11905–11910 [PubMed] [Google Scholar]
- 3. Barra-Bily L, et al. 2010. Proteomic alterations explain phenotypic changes in Sinorhizobium meliloti lacking the RNA chaperone Hfq. J. Bacteriol. 192:1719–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barra-Bily L, Pandey SP, Trautwetter A, Blanco C, Walker GC. 2010. The Sinorhizobium meliloti RNA chaperone Hfq mediates symbiosis of S. meliloti and alfalfa. J. Bacteriol. 192:1710–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berghoff BA, Glaeser J, Sharma CM, Vogel J, Klug G. 2009. Photooxidative stress-induced and abundant small RNAs in Rhodobacter sphaeroides. Mol. Microbiol. 74:1497–1512 [DOI] [PubMed] [Google Scholar]
- 6. Berghoff BA, et al. 2011. Contribution of Hfq to photooxidative stress resistance and global regulation in Rhodobacter sphaeroides. Mol. Microbiol. 80:1479–1495 [DOI] [PubMed] [Google Scholar]
- 7. Brantl S, Wagner EG. 1994. Antisense RNA-mediated transcriptional attenuation occurs faster than stable antisense/target RNA pairing: an in vitro study of plasmid pIP501. EMBO J. 13:3599–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan RG, Link TM. 2007. Hfq structure, function and ligand binding. Curr. Opin. Microbiol. 10:125–133 [DOI] [PubMed] [Google Scholar]
- 9. Caswell CC, et al. 2012. Identification of two small regulatory RNAs linked to virulence in Brucella abortus 2308. Mol. Microbiol. 85:345–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caswell CC, Gaines JM, Roop RM. 2012. The RNA chaperone Hfq independently coordinates expression of the VirB type IV secretion system and the LuxR-type regulator BabR in Brucella abortus 2308. J. Bacteriol. 194:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chao Y, Vogel J. 2010. The role of Hfq in bacterial pathogens. Curr. Opin. Microbiol. 13:24–33 [DOI] [PubMed] [Google Scholar]
- 12. Chesnokova O, Coutinho JB, Khan IH, Mikhail MS, Kado CI. 1997. Characterization of flagella genes of Agrobacterium tumefaciens, and the effect of a bald strain on virulence. Mol. Microbiol. 23:579–590 [DOI] [PubMed] [Google Scholar]
- 13. Chevrot R, et al. 2006. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U. S. A. 103:7460–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiang MK, Lu MC, Liu LC, Lin CT, Lai YC. 2011. Impact of Hfq on global gene expression and virulence in Klebsiella pneumoniae. PLoS One 6:e22248 doi:10.1371/journal.pone.0022248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Cleene M, De Ley J. 1976. The host range of crown gall. Bot. Rev. 42:389–466 [Google Scholar]
- 16. Ding Z, Christie PJ. 2003. Agrobacterium tumefaciens twin-arginine-dependent translocation is important for virulence, flagellation, and chemotaxis but not type IV secretion. J. Bacteriol. 185:760–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixon RA. 2001. Natural products and plant disease resistance. Nature 411:843–847 [DOI] [PubMed] [Google Scholar]
- 18. Dunoyer P, Himber C, Voinnet O. 2006. Induction, suppression and requirement of RNA silencing pathways in virulent Agrobacterium tumefaciens infections. Nat. Genet. 38:258–263 [DOI] [PubMed] [Google Scholar]
- 19. Fender A, Elf J, Hampel K, Zimmermann B, Wagner EGH. 2010. RNAs actively cycle on the Sm-like protein Hfq. Genes Dev. 24:2621–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folichon M, Allemand F, Regnier P, Hajnsdorf E. 2005. Stimulation of poly(A) synthesis by Escherichia coli poly(A)polymerase I is correlated with Hfq binding to poly(A) tails. FEBS J. 272:454–463 [DOI] [PubMed] [Google Scholar]
- 21. Folichon M, et al. 2003. The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res. 31:7302–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franze de Fernandez MT, Hayward WS, August JT. 1972. Bacterial proteins required for replication of phage Q ribonucleic acid. Purification and properties of host factor I, a ribonucleic acid-binding protein. J. Biol. Chem. 247:824–831 [PubMed] [Google Scholar]
- 23. Galibert F, et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668–672 [DOI] [PubMed] [Google Scholar]
- 24. Gao M, Barnett MJ, Long SR, Teplitski M. 2010. Role of the Sinorhizobium meliloti global regulator Hfq in gene regulation and symbiosis. Mol. Plant Microbe Interact. 23:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geng J, et al. 2009. Involvement of the post-transcriptional regulator Hfq in Yersinia pestis virulence. PLoS One 4:e6213 doi:10.1371/journal.pone.0006213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldberg AL, Moerschell RP, Chung CH, Maurizi MR. 1994. ATP-dependent protease La (lon) from Escherichia coli. Methods Enzymol. 244:350–375 [DOI] [PubMed] [Google Scholar]
- 27. Gottesman S, Storz G. 2011. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb. Perspect. Biol. 3:a003798 doi:10.1101/cshperspect.a003798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottwein E, et al. 2007. A viral microRNA functions as an orthologue of cellular miR-155. Nature 450:1096–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gripenland J, et al. 2010. RNAs: regulators of bacterial virulence. Nat. Rev. Microbiol. 8:857–866 [DOI] [PubMed] [Google Scholar]
- 30. Guisbert E, Rhodius VA, Ahuja N, Witkin E, Gross CA. 2007. Hfq modulates the sigmaE-mediated envelope stress response and the sigma32-mediated cytoplasmic stress response in Escherichia coli. J. Bacteriol. 189:1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hopkins JF, Panja S, Woodson SA. 2011. Rapid binding and release of Hfq from ternary complexes during RNA annealing. Nucleic Acids Res. 39:5193–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hwang HH, et al. 2010. Agrobacterium-produced and exogenous cytokinin-modulated Agrobacterium-mediated plant transformation. Mol. Plant Pathol. 11:677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahng LS, Shapiro L. 2001. The CcrM DNA methyltransferase of Agrobacterium tumefaciens is essential, and its activity is cell cycle regulated. J. Bacteriol. 183:3065–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klüsener S, et al. 2010. Proteomic and transcriptomic characterization of a virulence-deficient phosphatidylcholine-negative Agrobacterium tumefaciens mutant. Mol. Genet. Genomics 283:575–589 [DOI] [PubMed] [Google Scholar]
- 35. Langklotz S, Schäkermann M, Narberhaus F. 2011. Control of lipopolysaccharide biosynthesis by FtsH-mediated proteolysis of LpxC is conserved in enterobacteria but not in all Gram-negative bacteria. J. Bacteriol. 193:1090–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Latch JN, Margolin W. 1997. Generation of buds, swellings, and branches instead of filaments after blocking the cell cycle of Rhizobium meliloti. J. Bacteriol. 179:2373–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee T, Feig AL. 2008. The RNA binding protein Hfq interacts specifically with tRNAs. RNA 14:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Link TM, Valentin-Hansen P, Brennan RG. 2009. Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc. Natl. Acad. Sci. U. S. A. 106:19292–19297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. 1998. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. U. S. A. 95:12462–12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mikulecky PJ, et al. 2004. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat. Struct. Mol. Biol. 11:1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mysore KS, et al. 1998. Role of the Agrobacterium tumefaciens VirD2 protein in T-DNA transfer and integration. Mol. Plant Microbe Interact. 11:668–683 [DOI] [PubMed] [Google Scholar]
- 42. Papenfort K, Vogel J. 2010. Regulatory RNA in bacterial pathogens. Cell Host Microbe 8:116–127 [DOI] [PubMed] [Google Scholar]
- 43. Paulick A, et al. 2009. Two different stator systems drive a single polar flagellum in Shewanella oneidensis MR-1. Mol. Microbiol. 71:836–850 [DOI] [PubMed] [Google Scholar]
- 44. Pitzschke A, Hirt H. 2010. New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J. 29:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Porath J. 1992. Immobilized metal ion affinity chromatography. Protein Expr. Purif. 3:263–281 [DOI] [PubMed] [Google Scholar]
- 46. Repoila F, Majdalani N, Gottesman S. 2003. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol. Microbiol. 48:855–861 [DOI] [PubMed] [Google Scholar]
- 47. Robertson GT, Roop RM., Jr 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:690–700 [DOI] [PubMed] [Google Scholar]
- 48. Schägger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 48a. Schmidt-Eisenlohr H, et al. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 181:7485–7492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. 2002. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 21:3546–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma CM, et al. 2011. Pervasive post-transcriptional control of genes involved in amino acid metabolism by the Hfq-dependent GcvB small RNA. Mol. Microbiol. 81:1144–1165 [DOI] [PubMed] [Google Scholar]
- 51. Simonsen KT, et al. 2011. A role for the RNA chaperone Hfq in controlling adherent-invasive Escherichia coli colonization and virulence. PLoS One 6:e16387 doi:10.1371/journal.pone.0016387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sittka A, et al. 2008. Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet. 4:e1000163 doi:10.1371/journal.pgen.1000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sittka A, Pfeiffer V, Tedin K, Vogel J. 2007. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol. Microbiol. 63:193–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sobrero P, Valverde C. 2011. Evidences of autoregulation of hfq expression in Sinorhizobium meliloti strain 2011. Arch. Microbiol. 193:629–639 [DOI] [PubMed] [Google Scholar]
- 55. Sonnleitner E, Schuster M, Sorger-Domenigg T, Greenberg EP, Blasi U. 2006. Hfq-dependent alterations of the transcriptome profile and effects on quorum sensing in Pseudomonas aeruginosa. Mol. Microbiol. 59:1542–1558 [DOI] [PubMed] [Google Scholar]
- 56. Soper TJ, Doxzen K, Woodson SA. 2011. Major role for mRNA binding and restructuring in sRNA recruitment by Hfq. RNA 17:1544–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Soper TJ, Woodson SA. 2008. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA 14:1907–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Su S, Stephens BB, Alexandre G, Farrand SK. 2006. Lon protease of the alpha-proteobacterium Agrobacterium tumefaciens is required for normal growth, cellular morphology and full virulence. Microbiology 152:1197–1207 [DOI] [PubMed] [Google Scholar]
- 59. Subramanian S, et al. 2008. Novel and nodulation-regulated microRNAs in soybean roots. BMC Genomics 9 doi:10.1186/1471-2164-9-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Torres-Quesada O, et al. 2010. The Sinorhizobium meliloti RNA chaperone Hfq influences central carbon metabolism and the symbiotic interaction with alfalfa. BMC Microbiol. 10:71 doi:10.1186/1471-2180-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsai YL, et al. 2009. Small heat-shock protein HspL is induced by VirB protein (s) and promotes VirB/D4-mediated DNA transfer in Agrobacterium tumefaciens. Microbiology 155:3270–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsui HC, Leung HC, Winkler ME. 1994. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol. Microbiol. 13:35–49 [DOI] [PubMed] [Google Scholar]
- 63. Valentin-Hansen P, Eriksen M, Udesen C. 2004. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol. Microbiol. 51:1525–1533 [DOI] [PubMed] [Google Scholar]
- 64. Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 9:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Voss B, et al. 2009. Expression of small RNAs in Rhizobiales and protection of a small RNA and its degradation products by Hfq in Sinorhizobium meliloti. Biochem. Biophys. Res. Commun. 390:331–336 [DOI] [PubMed] [Google Scholar]
- 66. Waters LS, Storz G. 2009. Regulatory RNAs in bacteria. Cell 136:615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wessel M, et al. 2006. Virulence of Agrobacterium tumefaciens requires phosphatidylcholine in the bacterial membrane. Mol. Microbiol. 62:906–915 [DOI] [PubMed] [Google Scholar]
- 68. Wilms I, Overlöper A, Nowrousian M, Sharma CM, Narberhaus F. 2012. Deep sequencing uncovers numerous small RNAs on all four replicons of the plant pathogen Agrobacterium tumefaciens. RNA Biol. 9:446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wilms I, Voss B, Hess WR, Leichert LI, Narberhaus F. 2011. Small RNA-mediated control of the Agrobacterium tumefaciens GABA binding protein. Mol. Microbiol. 80:492–506 [DOI] [PubMed] [Google Scholar]
- 70. Wood DW, et al. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317–2323 [DOI] [PubMed] [Google Scholar]
- 71. Wright R, Stephens C, Shapiro L. 1997. The CcrM DNA methyltransferase is widespread in the alpha subdivision of proteobacteria, and its essential functions are conserved in Rhizobium meliloti and Caulobacter crescentus. J. Bacteriol. 179:5869–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zaher HS, Green R. 2010. Hyperaccurate and error-prone ribosomes exploit distinct mechanisms during tRNA selection. Mol. Cell 39:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang A, et al. 2003. Global analysis of small RNA and mRNA targets of Hfq. Mol. Microbiol. 50:1111–1124 [DOI] [PubMed] [Google Scholar]
- 74. Zhu Y, Nam J, Carpita NC, Matthysse AG, Gelvin SB. 2003. Agrobacterium-mediated root transformation is inhibited by mutation of an Arabidopsis cellulose synthase-like gene. Plant Physiol. 133:1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zupan J, Muth TR, Draper O, Zambryski P. 2000. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 23:11–28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.