Abstract
Conversion of acetate to methane (aceticlastic methanogenesis) is an ecologically important process carried out exclusively by methanogenic archaea. An important enzyme for this process as well as for methanogenic growth on carbon monoxide is the five-subunit archaeal CO dehydrogenase/acetyl coenzyme A (CoA) synthase multienzyme complex (CODH/ACS) catalyzing both CO oxidation/CO2 reduction and cleavage/synthesis of acetyl-CoA. Methanosarcina acetivorans C2A contains two very similar copies of a six-gene operon (cdh genes) encoding two isoforms of CODH/ACS (Cdh1 and Cdh2) and a single CdhA subunit, CdhA3. To address the role of the CODH/ACS system in M. acetivorans, mutational as well as promoter/reporter gene fusion analyses were conducted. Phenotypic characterization of cdh disruption mutants (three single and double mutants, as well as the triple mutant) revealed a strict requirement of either Cdh1 or Cdh2 for acetotrophic or carboxidotrophic growth, as well as for autotrophy, which demonstrated that both isoforms are bona fide CODH/ACS. While expression of the Cdh2-encoding genes was generally higher than that of genes encoding Cdh1, both appeared to be regulated differentially in response to growth phase and to changing substrate conditions. While dispensable for growth, CdhA3 clearly affected expression of cdh1, suggesting that it functions in signal perception and transduction rather than in catabolism. The data obtained argue for a functional hierarchy and regulatory cross talk of the CODH/ACS isoforms.
INTRODUCTION
Methanogenic archaea play a pivotal role in the global carbon cycle because they catalyze the terminal step in anaerobic biomass degradation in the absence of exogenous electron acceptors. While most methanogens are very limited in the range of the substrates they can convert to methane and can use only H2+CO2 or formate, which is reduced to methane via the pathway of hydrogenotrophic methanogenesis, Methanosarcina species are more versatile in their energy metabolism and can use, beside H2+CO2, methylated compounds and acetate as growth substrates (9, 46). Acetate is first activated to acetyl coenzyme A (acetyl-CoA) (12) and subsequently cleaved into a methyl group, an enzyme-bound carbonyl, and free CoA by the archaeal five-subunit carbon monoxide dehydrogenase/acetyl-CoA synthase multienzyme complex (CODH/ACS), also designated acetyl-CoA decarbonylase synthase (ACDS) (16, 17). The methyl group is transferred by CODH/ACS to the C1-transferring cofactor tetrahydrosarcinapterin and subsequently reduced to methane (13). The reducing equivalents are derived from the oxidation of the carbonyl group to CO2, but how the electrons are transferred is currently unclear.
Of the few methanogens for which carbon monoxide (CO)-dependent growth could be demonstrated (32), M. acetivorans is unique, as it does not produce H2, due to the absence of hydrogenase activity. Instead, it produces, beside methane, substantial amounts of formate and acetate (37), as well as small amounts of methyl sulfides from CO (31, 33). Previous genetic analysis suggests that acetate formation from CO proceeds via acetyl-CoA and, thus, via a pathway analogous to the reductive acetyl-CoA (the Wood-Ljungdahl) pathway, the central energy-conserving pathway of acetogenic bacteria (37). Key to this pathway is again CODH/ACS, combining a methyl group formed in the CO2 reduction pathway, CO and CoA. Acetyl-CoA is subsequently converted via acetyl-phosphate to acetate, a process that is catalyzed by phosphotransacetylase and acetate kinase, which allows energy conservation by substrate-level phosphorylation. Autotrophic carbon assimilation in methanogens also proceeds via the reductive acetyl-CoA pathway, involving the archaeal five-subunit CODH/ACS multienzyme complex (17, 24).
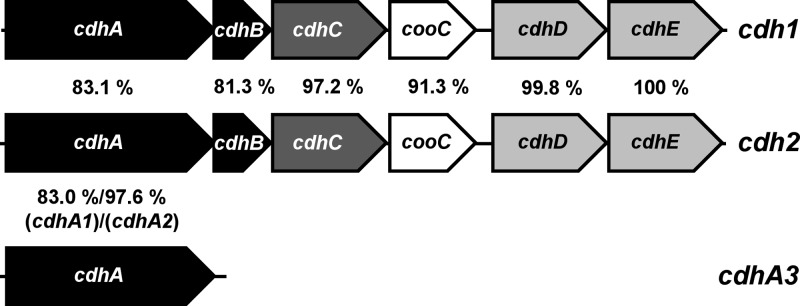
M. acetivorans contains genes for five catalytic subunits of CODH (15). The genes (ma1309 and ma3282), encoding two orthologs of the homodimeric CODH, first characterized in anaerobic CO-oxidizing bacteria (22), were shown not to be required for growth on any substrate; however, a mutant lacking both isoforms grew more slowly on CO than the wild type, indicating a role in carboxidotrophic growth of the organism (39). Furthermore, two archaeal five-subunit CODH/ACS isoforms (encoded by cdh genes), termed Cdh1 (MA1016-MA1011) and Cdh2 (MA3860-MA3865) (39), which are encoded in putative transcriptional units (Fig. 1), and a single gene encoding a CdhA subunit, referred to as CdhA3 (MA4399) (Fig. 1), are also present in M. acetivorans. So far, no function has been assigned to CdhA3. Proteome analyses showed that both isoforms of the five-subunit enzyme are abundant in M. acetivorans when acetate or CO serves as the sole source of energy (26, 39), but whether the two isoforms have discrete functions is not clear. Qualitative analysis of CODH/ACS-encoding transcripts of Methanosarcina mazei Gö1 led to the conclusion that one CODH/ACS isoform may be involved in carbon fixation, while the other may be responsible for acetate catabolism (11). Methanosarcina thermophila, an acetotrophic methanogen, was shown to contain only one isoform of CODH/ACS, which therefore has to function in both anabolic acetyl-CoA synthesis and catabolic acetyl-CoA cleavage (18).
Fig 1.
Comparison of the cdh isogenes in M. acetivorans. The two putative operons encoding CODH/ACS, designated cdh1 (ma1016-ma1011) and cdh2 (ma3860-ma3865), as well as cdhA3 (ma4399), are shown. Genes for CODH subunits are in black, those for ACS are in dark gray, those for corrinoid-containing methyltransferase are in light gray, and those for the cooC (encoding the putative nickel insertase) are in white; percentages are amino acid identities between the respective deduced Cdh subunits.
In this study, we addressed the function and regulation of the CODH/ACS-encoding genes in M. acetivorans. Phenotypic analyses of mutants carrying deletions in the Cdh-encoding genes show that autotrophic growth, as well as utilization of either acetate or CO, requires the presence of at least one CODH/ACS. This finding unequivocally demonstrates that both isoforms are bona fide CODH/ACS capable of playing both anabolic and catabolic roles. Furthermore, we show by analyzing promoter/reporter gene fusions in different genetic backgrounds, under different growth conditions, and in different growth phases that the Cdh1 and Cdh2 encoding genes are differentially regulated, particularly during the shift from one energy substrate to another, which suggests discrete but overlapping physiological functions of the two isoforms. Surprisingly, our data point to a role for CdhA3 in the substrate-dependent signal transduction pathways involved in transcriptional regulation of the Cdh-encoding genes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli was grown under standard conditions (48). M. acetivorans strains, described in Table 1, were grown in HS medium as described previously (44). Either methanol (125 mM), sodium acetate (120 mM), or CO (150 kPa) served as the energy source for growth. Adaptation of M. acetivorans to CO was carried out as described previously (37). When they had been growing on a substrate for at least 15 generations, cultures were considered fully adapted. For a shift from methanol to acetate as the growth substrate, cells of a culture grown on methanol were either directly transferred (10% inoculum) into medium containing only acetate as the energy source (growth curves of cdh mutants) or harvested by centrifugation, washed twice in substrate-free medium to avoid carryover of methanol, and transferred to acetate-containing medium (reporter gene expression analysis). When required for methylotrophic growth of M. acetivorans mutants, the medium was supplemented either with 40 mM sodium acetate or 100 mM pyruvate. Solid medium contained 1.5% (wt/vol) Bacto agar (4). For selection of M. acetivorans strains carrying the puromycin transacetylase gene (pac), puromycin (CalBiochem, San Diego, CA) was added from sterile, anaerobic stocks at a final concentration of 2 μg/ml. The purine analog 8-aza-2,6-diaminopurine (8-ADP; Sigma, St. Louis, MO) was added from sterile, anaerobic stocks at a final concentration of 20 μg/ml for selection against the hypoxanthine phosphoribosyl transferase gene (hpt). Growth of M. acetivorans was monitored by assessing optical density at 578 nm (OD578) using a spectrophotometer (Genesys 10vis; Thermo, Dreieich, Germany). Cell titer and size were also determined by phase-contrast microscopy using a Thoma chamber and micrometer-engraved oculars.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype, description, and/or constructiona | Reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH10B | General cloning | Invitrogen |
| WM1788 | pir+ for replication of R6K derivatives | 20 |
| M. acetivorans | ||
| C2A | Wild type | 43 |
| WWM1 | C2A, Δhpt | 34 |
| MCD1 | WWM1, Δcdh1::frt | This study |
| MCD2 | WWM1, Δcdh2::frt | This study |
| MCD3 | WWM1, ΔcdhA3::frt | This study |
| MCD31 | MCD3, Δcdh1::frt | This study |
| MCD32 | MCD3, Δcdh2::frt | This study |
| MCD21 | MCD2, Δcdh1::frt | This study |
| MCD213 | MCD21, ΔcdhA3::frt | This study |
| P1016 | C2A, Δhpt::cdhA1p-uidA | This study |
| P3860 | C2A, Δhpt::cdhA2p-uidA | This study |
| P4399 | C2A, Δhpt::cdhA3p-uidA | This study |
| P1016D2 | MCD2, Δhpt::cdhA1p-uidA (integration at Δhpt locus using pKY1016) | This study |
| P1016D3 | P1016, ΔcdhA3::frt | This study |
| P3860D1 | P3860, Δcdh1::frt | This study |
| P3860D3 | P3860, ΔcdhA3::frt | This study |
| Plasmids | ||
| pJK301 | pBluescript based; contains Frt-pac-hpt-Frt to construct disruption cassette | 49 |
| pMP44 | Vector for markerless deletion of genes in M. acetivorans; oriR6K; encodes hpt | 34 |
| pUP1016 | ma1016 upstream PCR (using primers 5-up1016 and 3-up1016) cloned into pJK301 via XhoI/HindIII | This study |
| pKoCD1 | ma1011 downstream PCR fragment (using primers 5-do1011 and 3-do1011) cloned into pUP1016 via BamHI/SpeI | This study |
| pUP3860 | ma3860 upstream PCR (using primers 5-up3860 and 3-up3860) cloned into pJK301 via XhoI/HindIII | This study |
| pKoCD2 | ma3865 downstream PCR fragment (using primers 5-do3865 and 3-do3865) cloned into pUP3860 via BamHI/SpeI | This study |
| pUP4399 | ma4399 upstream PCR (using primers 5-up4399 and 3-up4399) cloned into pJK301 via XhoI/HindIII | This study |
| pKoCD3 | ma4399 downstream PCR fragment (using primers 5-do4399 and 3-do4399) cloned into pUP4399 via BamHI/SpeI | This study |
| pMR55 | Encodes Flp recombinase | 49 |
| pMR51 | Vector for insertion of uidA fusions into hpt locus of M. acetivorans; oriR6K | 6 |
| pP1016 | cdhA1p PCR (using primers oP1016for and oP1016rev) cloned into pMR51 5′ of uidA via NdeI/NheI | This study |
| pP3860 | cdhA2p PCR (using primers oP3860for and oP3860rev) cloned into pMR51 5′ of uidA via NdeI/NheI | This study |
| pP4399 | cdhA3p PCR (using primers oP4399for and oP3860rev) cloned into pMR51 5′ of uidA via NdeI/NheI | This study |
| pKY1016 | AflII/ApaI fragment (containing cdhA1p-uidA fusion) of pP1016 blunted, cloned into NruI site of pMP44 | This study |
Details of construction and plasmid maps are available upon request; the oligonucleotides used are listed in Table S1 in the supplemental material.
Molecular methods, plasmid construction, and transformation.
Standard molecular methods were used for manipulation of plasmid DNA from E. coli (3). Plasmids used are presented in Table 1. All plasmids in this study are nonreplicating in Methanosarcina. Genomic DNA from M. acetivorans was isolated using a modified cetyl trimethylammonium bromide-NaCl method (30). Genomic DNA of M. acetivorans was used as the template for PCR or subjected to Southern hybridization using a digoxigenin (DIG) system (Roche) (34) to verify the genotypes of M. acetivorans cdh mutants. For Southern hybridization chromosomal DNA of M. acetivorans was isolated, restricted with EcoRV, separated electrophoretically, transferred onto a nylon membrane, and treated with one DIG-labeled DNA fragment as a probe. The probe was generated by PCR using ocdhA-SB/forw and ocdhA-SB/rev (see Table S1 in the supplemental material), which corresponds to approximately 540 bp of cdhA1 but hybridizes to each of the three cdhA genes. DNA sequences of all cloning intermediates employing PCR were confirmed by sequencing at SRD Biotech (Bad Homburg) using the BigDye terminator cycle sequencing protocol (Applied Biosystems, Foster City, CA). E. coli was transformed by electroporation (14). Liposome-mediated transformation was used for Methanosarcina species as described previously (29), with modifications (4).
Construction of M. acetivorans cdh mutants.
Approximately 1-kb regions flanking the first (ma1016 and ma3860) and last (ma1011 and ma3865) genes of the two cdh operons, or flanking cdhA3 (ma4399), were amplified by PCR and cloned into pJK301 (19). Thus, gene disruption constructs were created in which the Frt-pac-hpt-Frt cassette was flanked by M. acetivorans DNA for homologous recombination. The resulting plasmids pKoCD1 (to delete cdh1, ma1016 to ma1011), pKoCD2 (to delete cdh2, ma3860 to ma3865), and pKoCD3 (to delete cdhA3, ma4399) (Table 1) were linearized using AscI and transferred into M. acetivorans WWM1 (Table 1). There, a double homologous recombination event replaced the respective target gene or operon with the Frt-pac-hpt-Frt cassette, selected for by puromycin. Subsequent transformation of the respective mutant strain with pMR55 (38), and thus, transient synthesis of Flp recombinase led to elimination of the region flanked by the Frt sites, selected for by 8-ADP. In this way, unmarked chromosomal cdh lesions were created with only a single Frt site remaining. The resulting mutants were MCD1 (cdh1 deleted), MCD2 (cdh2 deleted), and MCD3 (cdhA3 deleted). The same procedure was applied to the cdh single mutants to generate cdh double mutants: pKoCD1 was used to create MCD21 from MCD2 and to create MCD31 from MCD3 (Table 1); the latter strain was used to create MCD32 using pKoCD2; the double mutant MCD21 was used to create the triple mutant MCD213 using pKoCD3 (Table 1).
Promoter/reporter gene fusion analysis.
To analyze expression from the promoter regions preceding the three cdhA genes of M. acetivorans approximately 1 kb of the genomic region upstream of the respective gene (ma1016 for cdhA1; ma3860 for cdhA2; ma4399 for cdhA3) was amplified by PCR. At the 3′ end of the fragment, an NdeI site was introduced which overlaps the predicted translation start codon of the corresponding cdhA gene; the respective DNA fragment was cloned into pMR51, thereby creating a transcriptional/translational fusion of the respective cdhA promoter region and uidA with its translational start codon superimposed on that of the corresponding cdhA gene. Thus, β-glucuronidase activity in these strains can be used as a measure of cdhA transcription and translation initiation. These reporter gene fusions were inserted into the hpt locus of M. acetivorans C2A via the markerless insertion procedure (34). The resulting strains were designated P1016 (to assess cdhA1 expression), P3860 (to assess cdhA2 expression), and P4399 (to assess cdhA3 expression) (Table 1). Reporter strains carrying deletions of the cdh loci were created by deleting the respective operon or gene in either P1016 or P3860 (Table 1) or by inserting a reporter gene fusion into the hpt locus of a cdh mutant (strain P1016D2) (Table 1).
Batch cultures (300 ml) were grown in 1-liter Müller-Krempel bottles and analyzed for growth phase-dependent β-glucuronidase activity. The cultures used were fully adapted to methanol, acetate, or CO, or methanol-adapted cultures were shifted to acetate (see above). Samples were taken during the whole course of growth and analyzed for β-glucuronidase activity. Briefly, cells were harvested by centrifugation and osmotically lysed by addition of 50 mM Tris HCl buffer, pH 8.0, containing 1 mM dithiothreitol, 0.1 μg/ml DNase I, and 0.1 μg/ml RNase A. The lysate was cleared by centrifugation, and the specific activity of β-glucuronidase was determined by monitoring cleavage of p-nitrophenol-glucuronide at 415 nm as described previously (34). Protein concentration was determined by the method of Bradford (7) using bovine serum albumin as the standard.
For single-point, steady-state determination of β-glucuronidase activity, 10-ml cultures (5 ml for growth on CO) (five experimental replicates) were grown in Balch tubes, and the cells in the parallel cultures were harvested at exactly the same optical density in the early exponential growth phase (OD578, 0.2 to 0.25 for CO and acetate and 0.4 for methanol). Samples were processed as described above.
Determination of CODH activity.
All manipulations to determine CODH activity were carried out under strictly anaerobic conditions using gas-tight vials or inside an anaerobic glove box (Coy, Grass Lake, MI) containing N2-H2 (96:4 [vol/vol]). Crude extract of M. acetivorans was prepared from cultures at early exponential growth phase at the same optical densities as for β-glucuronidase determination (see above). Cells were harvested by centrifugation and lysed in assay buffer (50 mM potassium phosphate, pH 7.2; 2 mM dithiothreitol) containing 0.1 μg/ml DNase I and 0.1 μg/ml RNase A, for 30 min. The lysate was cleared by centrifugation at 6,000 × g, and the supernatant was used for enzymatic assays. CODH activity was determined by monitoring CO-dependent reduction of methylviologen (MV) at 603 nm (ε603 = 11.3 mM−1 cm−1) as described previously (35), except that 8 mM MV was used and sodium dithionite and 2-mercaptoethanol were omitted. Nonspecific MV reduction activity was measured independently by omitting an electron donor and was used for correction of the specific CODH activity. Protein concentration was determined as described above.
RESULTS
Genotypic and phenotypic analysis of M. acetivorans cdh mutants.
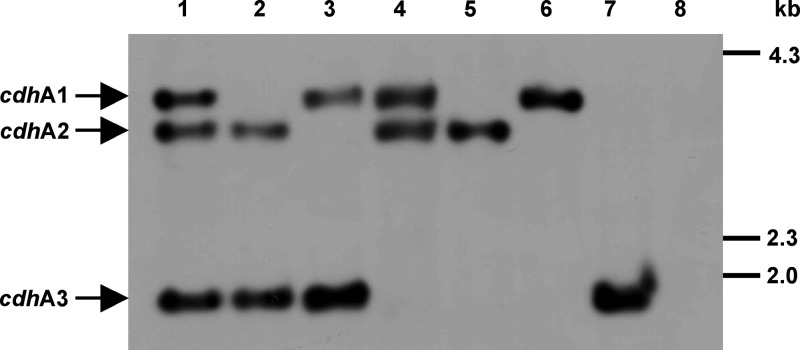
In order to assess the in vivo role of the two CODH/ACS isoforms, encoded by ma1016-ma1011 (Cdh1) and ma3860-ma3865 (Cdh2), as well as of the single CdhA3 subunit, encoded by ma4399, the encoding genes were deleted from the M. acetivorans chromosome by using the markerless deletion/disruption technique (38). Of the seven strains constructed, MCD1 lacks the entire cdh1 region (i.e., ma1016 through ma1011), MCD2 lacks the entire cdh2 region (i.e., ma3860 through ma3865), and MCD3 lacks cdhA3 (ma4399). The double mutants and the triple mutant were derived from these strains (see Materials and Methods), and their genotypes were verified by Southern hybridization. The sizes and pattern of the chromosomal fragments hybridizing with the probe (Fig. 2) are fully consistent with in silico predictions (3,729, 3,336, and 1,783 bp for the cdh1, cdh2, and cdhA3 loci, respectively), which confirmed the nature of the seven cdh mutants.
Fig 2.
Verification of the genotypes of cdh mutants via Southern hybridization. Restricted genomic DNA was probed with a DIG-labeled DNA fragment hybridizing with all three cdhA alleles. Lanes: 1, wild type (WWM1); 2, MCD1; 3, MCD2; 4, MCD3; 5, MCD31; 6, MCD32; 7, MCD21; 8, MCD213. Size standards are shown on the right.
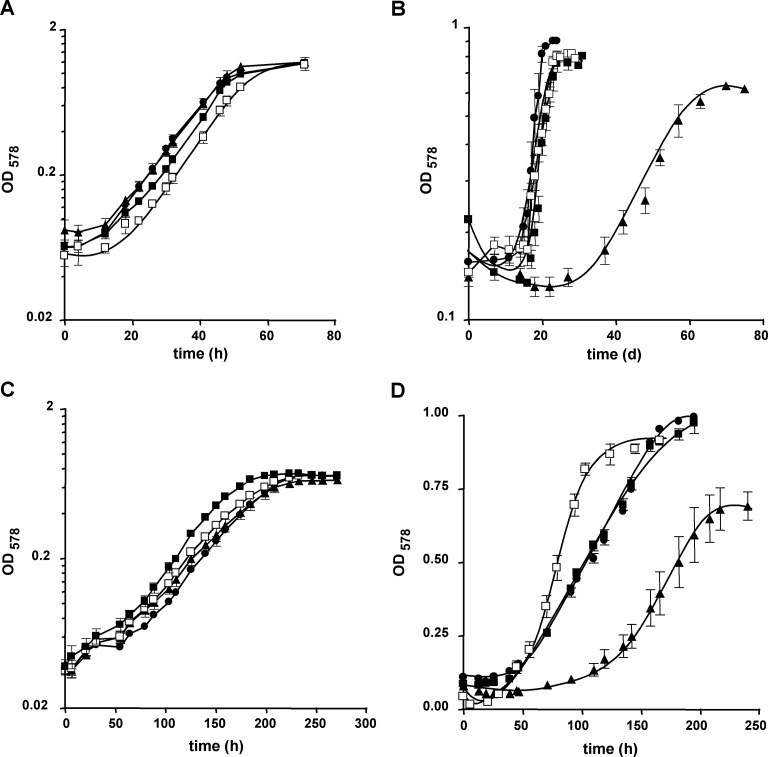
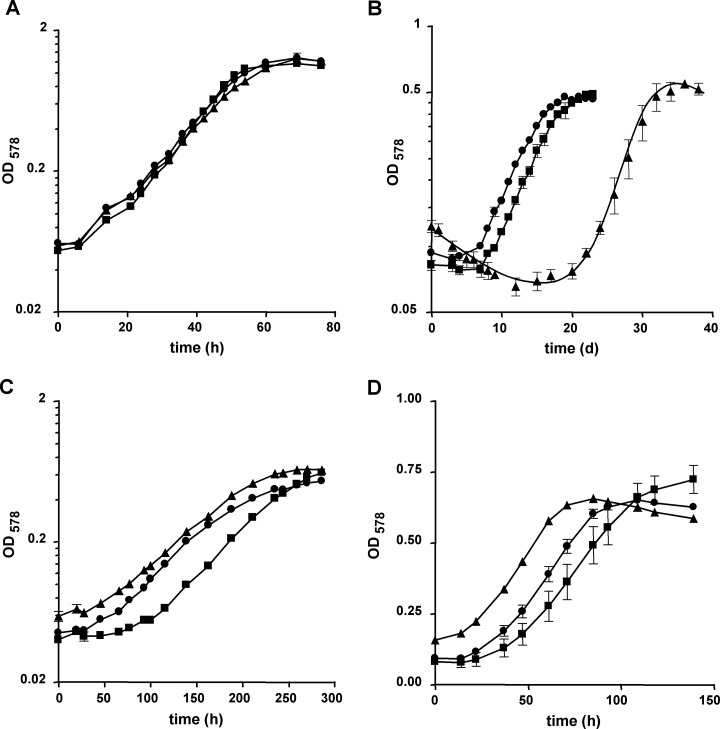
The consequences for M. acetivorans of the loss of one, two, or all three cdh loci were assessed by quantifying growth of the mutant strains with either methanol, acetate, or CO as the sole source of energy (Fig. 3 and 4; also, see Table S2 in the supplemental material). Growth on methanol was not affected in the cdh mutants, as long as either cdh1 or cdh2 was present (Fig. 3A and 4A). The same was observed when acetate was the growth substrate: neither the growth rate nor the final optical density reached by the mutants differed significantly from that of the wild type (Fig. 3C and 4C; also, see Table S2) when the cultures had been adapted to this substrate for at least 15 generations and provided that one of the cdh operons was still present on the chromosome. However, when shifted from methanol to acetate by inoculation of a methanol-grown culture into acetate-containing medium, the strains lacking cdh2 and still containing cdh1, irrespective of whether cdhA3 was also absent (strains MCD2 and MCD32), displayed a lag phase prolonged by approximately 10 days compared to the strains containing cdh2 (Fig. 3B and 4B). The transient decrease in optical density during the adaptation period resulted from both a decrease in cell titer, probably due to cell lysis, and a decrease in average cell size, probably resulting from reductive cell division, as evidenced by microscopy (data not shown). These data suggest that Cdh2 plays a more important role during the shift from methylotrophic to aceticlastic conditions than Cdh1, but also that loss of Cdh2 is fully compensated during continuous growth on acetate. However, the data allow no firm conclusion as to how the fitness of the mutants lacking Cdh2 for growth on acetate is improved. When strains were grown on CO, loss of Cdh1 (strains MCD1 and MCD31) had no obvious effect on growth (Fig. 3D and 4D), but the absence of Cdh2 (strain MCD2) resulted in a significantly reduced growth rate compared to the wild type (Fig. 3D), indicating that under this condition also, Cdh2 plays a more important role than Cdh1. While loss of CdhA3 alone had no adverse effect on either methylotrophic or aceticlastic growth, strikingly, the same mutation appeared to enable M. acetivorans to grow faster than the wild type on CO (strain MCD3) (Fig. 3D) and to compensate for loss of Cdh2 (strain MCD32) (Fig. 4D). These data strongly indicate that in the wild type, CdhA3 exerts an effect on carboxidotrophic growth by somehow negatively affecting Cdh1.
Fig 3.
Substrate-dependent growth of M. acetivorans cdh single mutants. Growth of the strains in the presence of methanol (A), during the shift from methanol to acetate (B; the zero time point indicates the transfer of the culture), in the presence of acetate (C), and in the presence of CO (D) was monitored by measuring the OD578. Filled squares, wild type (WWM1); filled circles, MCD1; filled triangles, MCD2; open squares, MCD3. Values are averages and standard deviations from at least three independent cultures; the experiments were qualitatively reproduced at least once. Doubling times and final optical densities are listed in Table S2 in the supplemental material.
Fig 4.
Substrate-dependent growth of M. acetivorans cdh double mutants. Growth of the strains in the presence of methanol (A), during the shift from methanol to acetate (B; the zero time point indicates the transfer of the culture), in the presence of acetate (C), and in the presence of CO (D) was monitored by measuring OD578. Squares, wild type (WWM1); circles, MCD31; triangles, MCD32. Values are averages and standard deviations from at least three independent cultures; the experiments were qualitatively reproduced at least once. Doubling times and final optical densities are listed in Table S2 in the supplemental material.
Both putative cdh operons encode bona fide CODH/ACS isoforms in M. acetivorans.
Regardless of the growth substrate and of the presence or absence of CdhA3, either Cdh1 or Cdh2 had to be present to enable the mutant to grow autotrophically. When both cdh operons had been deleted (cdh1 cdh2 double mutant MCD21 and cdh triple mutant MCD213), methylotrophic growth of the respective strains was strictly dependent on the presence of acetate or pyruvate in the medium (data not shown). Growth on methanol in the presence of 40 mM acetate was significantly slower in MCD21 and MCD213 than in the wild type (see Fig. S1 and Table S2 in the supplemental material), which may indicate that acetyl-CoA is not generated from exogenous acetate as efficiently as it is endogenously through the Wood-Ljungdahl pathway. Thus, either uptake or activation of acetate (via acetate kinase and phosphotransacetylase) could represent a growth-limiting step in these two strains. When MCD21 and MCD213 were shifted from a medium containing methanol plus acetate to a medium containing only acetate, no growth was observed (scored after 6 months of incubation [data not shown]). Also, MCD21 and MCD213 could not be adapted to grow with CO as the sole energy source (data not shown). Together with the phenotypes of the other cdh mutants, the data unequivocally demonstrate that in M. acetivorans both cdh1 and cdh2 encode bona fide CODH/ACS isoforms, one of which is required and sufficient for both catabolic acetyl-CoA cleavage (during aceticlastic growth) and anabolic acetyl-CoA synthesis (during CO2 fixation via the Wood-Ljungdahl pathway). The fact that the strains lacking both cdh1 and cdh2 could not be adapted to grow with CO indicates an important role for the CODH/ACS system under this condition, either via its acetyl-CoA-forming activity (which leads to acetate formation) or via its CO oxidizing activity, or both.
Differential expression of the cdh genes during growth.
Although principally able to complement each other, the growth phenotypes of the cdh mutants (see above) and previously reported differential abundances of subunits (26, 39) suggest discrete functions of the two CODH/ACS isoforms during growth on acetate and on CO. To assess the contribution of the isoforms to utilization of these substrates in the wild type, expression of the operons/genes encoding the CODH/ACS isoforms and CdhA3 was analyzed during growth in batch cultures. Since expression of highly homologous genes (Fig. 1) is not easily analyzed by PCR-based approaches, we chose reporter gene fusion analysis (see Materials and Methods). No significant β-glucuronidase activity (background level, approximately 0.5 mU/mg) could be measured in the strain carrying the cdhA3p-uidA fusion (P4399) under any of the conditions tested. Since the promoters for the cdhA genes have not been precisely mapped, we therefore only assumed that all required regulatory sequences were present within the 1-kb region upstream of the coding region. Thus, a plausible explanations for this observation is that the promoter region chosen did not contain all required expression signals, which could obviate uidA expression. Alternatively, the gene may not be expressed at a level sufficient to be detected with the method used here. Strain P4399 was therefore not analyzed further.
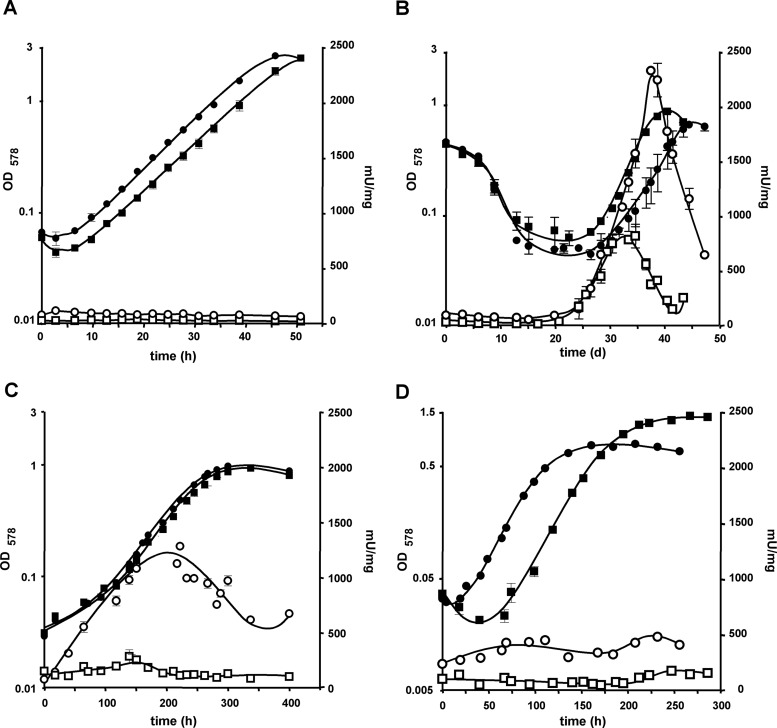
Methanol-dependent expression from the cdhA1 (strain P1016) and the cdhA2 (strain P3860) promoters was relatively low and remained constant throughout the different phases of growth during batch cultivation (Fig. 5A), which is in accordance with proteomic analyses (26, 39) and the anabolic role of CODH/ACS during methylotrophic growth. Under aceticlastic conditions (Fig. 5C), both cdhA genes were expressed at a much higher level, and their expression changed with the growth phases. While cdhA2 expression was lowest after dilution into fresh medium, it increased linearly during exponential growth, stagnating only in late-exponential growth phase and declining in stationary phase. On the other hand, cdhA1 expression increased only modestly (threefold) early in the exponential growth phase but was otherwise expressed at a rather constant level. When methanol-grown cells were shifted to medium containing only acetate as the sole source of energy (Fig. 5B) expression of both cdh promoters stagnated at the level found during growth on methanol for the whole adaptation period, which is typically 12 to 21 days (Fig. 3B and 4B). The number of washed cells pregrown on methanol and used for inoculation was rather high, to ensure that β-glucuronidase activity was significantly above the detection limit of the assay; notably, the time required for adapting to the new substrate was not affected by this measure (compare Fig. 3B, 4B, and 5B). Only when the cells started to grow did both cdhA promoters commence expression, which displayed a pattern similar to that of acetate-adapted cells. A remarkable difference, however, was the levels of expression, which were at least two- to threefold increased in both reporter strains. The higher levels of cdh expression could mean that larger amounts of CODH/ACS may be required during the substrate shift than under adapted conditions. Based on the levels of expression both during substrate shift and during growth on acetate after adaptation, it appears that Cdh2 is mainly responsible for acetyl-CoA cleavage during aceticlastic growth and that Cdh1 plays only a supplementary role. This conclusion is fully consistent with the observed growth behavior of the cdh2 mutants when shifted from methanol to acetate, where lack of Cdh2 leads to a dramatically prolonged lag phase (Fig. 3B and 4B).
Fig 5.
Growth- and substrate-dependent cdhAp-uidA expression in M. acetivorans. Cultures were grown on methanol (A), shifted from methanol to acetate (B), grown on acetate (C), or grown on CO (D); OD578 (filled symbols) indicates growth; β-glucuronidase activity (open symbols) indicates expression from the specific promoters. Squares, P1016 (cdhA1p-uidA); circles, P3860 (cdhA2p-uidA).
Interestingly, neither of the cdhA genes was as highly expressed during growth on CO (Fig. 5D) as during aceticlastic growth. Expression of cdhA2 increased during early exponential growth and declined somewhat during late exponential growth before a second peak in expression occurred at the beginning of stationary phase (Fig. 5D). Thus, expression of the Cdh2-encoding genes is dynamic and is probably influenced by various parameters, which change during batch cultivation. On the other hand, expression of cdhA1 remained at a rather constant level during carboxidotrophic growth, increasing only somewhat as the cells entered stationary growth phase.
CO oxidation in M. acetivorans is mainly catalyzed by the CODH/ACS system.
Based on the reporter gene expression data one would expect lower CODH activity in M. acetivorans during growth on CO than during growth on acetate. The phenotypes of the cdh mutants further indicate that loss of one Cdh Isoform can be compensated and that CdhA3 plays a role in this process. Substrate-dependent CODH activity was therefore analyzed by determining CO-dependent methylviologen reduction (see Materials and Methods) in the wild type and in the cdh mutants. As can be seen from Table 2, CODH activity in the wild type is lowest when the strain is grown on methanol, consistent with the anabolic role of the enzyme under this condition. The CODH activities of most cdh mutants were similar to that of the wild type under this condition, except that the cdh2 mutant MCD2 exhibited approximately 50% of the wild-type CODH activity. Notably, and fully consistent with the previous results, this reduction of CODH activity was compensated to wild-type levels when CdhA3 was also absent (strain MCD32). When both Cdh1 and Cdh2 were lacking (strains MCD21 and MCD213) CODH activity dropped to approximately 5% of that of the wild type, corroborating the notion that CODH/ACS is the major CO-oxidizing activity under this condition. The remaining CODH activity is probably that of the homodimeric CODH isoforms (MA1309 and MA3282). However, this activity is apparently not sufficient to enable the strains to grow on acetate or CO. CODH activity in the wild type was approximately 7- to 10-fold higher when CO or acetate served as the growth substrate than when methanol was the substrate (Table 2), consistent with the catabolic role of this enzyme during carboxidotrophic and aceticlastic growth. Interestingly, the difference in CODH activity between CO- and acetate-grown cells was not as high as expected from the cdhA gene expression analyses, indicating that transcription and enzyme activity do not correlate directly but that the latter is influenced by posttranscriptional events acting on the Cdh system.
Table 2.
Specific CODH activity of the cdh mutants
| Strain | Specific activity ona: |
||
|---|---|---|---|
| Methanol | CO | Acetate | |
| WWM1 | 446 ± 19 | 3,363 ± 275 | 5,807 ± 794 |
| MCD1 | 492 ± 73 | 2,349 ± 65 | 2,497 ± 478 |
| MCD2 | 177 ± 43 | 2,278 ± 328 | 3,318 ± 356 |
| MCD3 | 539 ± 78 | 4,705 ± 259 | 4,083 ± 627 |
| MCD31 | 395 ± 6 | 4,026 ± 560 | 3,231 ± 279 |
| MCD32 | 481 ± 14 | 4,759 ± 1,223 | 17,931 ± 2,718 |
| MCD21 | 25.3 ± 6.1 | ND | ND |
| MCD213 | 28.8 ± 8.4 | ND | ND |
CO-dependent methylviologen reduction (nmol min−1 mg−1) in crude extracts of strains fully adapted to the respective growth substrates. Values are averages ± standard deviations from at least two independent experiments conducted three times in parallel. ND, not detectable (the strain cannot grow on the substrate).
CODH activity during aceticlastic and carboxidotrophic growth was reduced in the cdh1 mutant (strain MCD1) compared to the wild type, which suggests that Cdh1 contributes significantly to overall CODH activity in M. acetivorans. However, this contribution is not sufficient to affect growth on acetate or CO negatively when it is lacking (Fig. 3). A reduction in CODH activity was also observed when Cdh2 was absent (strain MCD2), except that the reduction in CODH activity was more pronounced during growth on acetate, again arguing for a more prominent role of Cdh2 under aceticlastic than carboxidotrophic conditions. As MCD1 and MCD2 contained similar levels of CODH activity during growth on CO, it seemed unlikely that it was the reason for the slower growth of the latter under this condition. It also became apparent that the CODH activities of the strains MCD1 and MCD2 combined were larger than that of the wild type under any condition tested, i.e., that removing one of the Cdh isoform led to an increase in the activity of the other. CODH activity of the strain lacking CdhA3 (MCD3) was similar during aceticlastic and carboxidotrophic growth and significantly higher than that of the wild type growing on CO, which could explain why this mutant grew faster than its parental strain on this substrate (Fig. 3D). The mutant lacking both Cdh2 and CdhA3 (strain MCD32) contained a somewhat higher CODH activity than the wild type during growth on CO but significantly more than the Cdh2-deficient strain (MCD2), which probably allowed compensation of the growth defect seen in the latter strain (compare Fig. 3D and 4D). Again, this finding strongly suggests that CdhA3 exerts—directly or indirectly—a negative effect on the activity of Cdh1. This notion is emphasized by the finding that when MCD32 is grown on acetate, its CODH activity is increased approximately threefold compared to that of MCD2 or the wild type (Table 2).
CdhA3 influences expression of cdh isogenes.
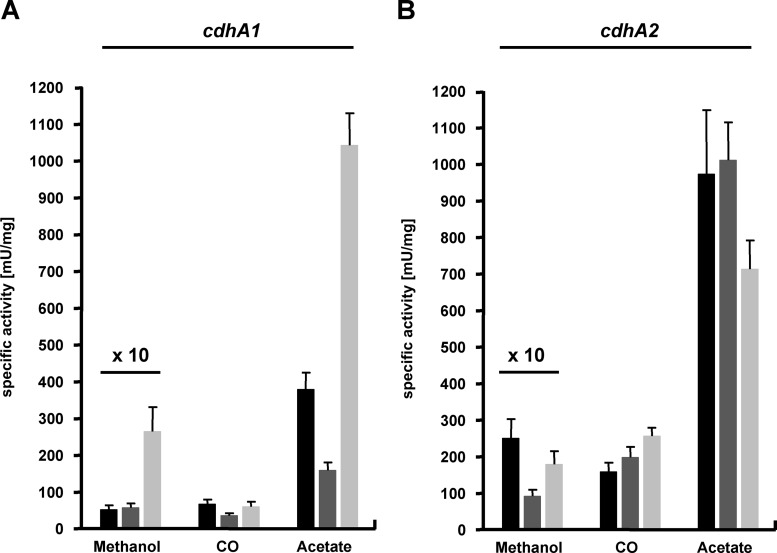
The CODH activities observed in the cdh mutants could not be plausibly explained by simple subtraction of isoform-specific activity from overall CODH activity. Instead, either different substrate-dependent catalytic efficiencies or different abundances of the isoforms, depending both on the growth substrate and on the mutant background, seemed to be the basis for the observations made. To investigate whether the two isoforms and/or CdhA3 influence expression of the other respective cdh genes, strains carrying, beside the reporter gene fusion, lesions of cdh1, cdh2, or cdhA3 were constructed (Table 1). We did not succeed in deleting cdh2 in strain P1016. Instead, an isogenic strain was constructed by inserting the cdhA1p-uidA fusion (Table 1) into the chromosomal hpt locus of the cdh2 mutant MCD2. Reporter activity in the respective single mutants during early exponential growth phase at one particular optical density was analyzed. The β-glucuronidase activities in the “wild-type” strains P1016 and P3860 (Fig. 6) qualitatively corresponded to the cdhA expression analyses during the course of growth (Fig. 5). The generally somewhat lower β-glucuronidase activities in this steady-state analysis might be due to the different cultivation scale (see Materials and Methods). While loss of Cdh1 barely affected expression of cdhA2 (Fig. 6B), the absence of Cdh2 resulted in an approximately 50% decrease of cdhA1 expression when CO or acetate served as the growth substrate (Fig. 6A). Furthermore, loss of cdhA3 did not affect cdhA1 expression during growth on CO but resulted in an almost-threefold increase in cdhA1 expression during growth on acetate (Fig. 6A). Conversely, loss of cdhA3 reduced expression of cdhA2 under the same conditions, if only by approximately 15% (Fig. 6B). Thus, CdhA3 exerts a negative effect on cdh1 transcription but a positive effect on cdh2 transcription, both of which appear to be acetate specific.
Fig 6.
Substrate-dependent cdhAp-uidA expression in cdh mutants of M. acetivorans. Cultures were grown on methanol, acetate, or CO; the cdhA1p-uidA fusion (left) was analyzed in the wild-type background (P1016, black bars), in the absence of cdh2 (P1016D2, dark gray bars), and in the absence of cdhA3 (P1016D3, light gray bars); the cdhA2p-uidA fusion (right) was analyzed in the wild-type background (P3860, black bars), in the absence of cdh1 (P3860D1, dark gray bars), and in the absence of cdhA3 (P3860D3, light gray bars). For clarity, all values obtained from cultivation on methanol were multiplied by a factor of 10.
DISCUSSION
Methanosarcina species metabolize a broader spectrum of substrates than other methanogenic archaea. Beside the amount of genetic inventory such a more generalistic lifestyle requires, Methanosarcina species are also known to often encode multiple homologs for numerous metabolic functions, which makes their genomes the largest of the Archaea (8, 15, 28). The selective advantage which retaining isoenzymes confers is not known for most cases, but it was shown that isoenzyme genes encoding, for example, catabolic methyltransferases are regulated in a sophisticated fashion (5, 6). In this study, we addressed the function and regulation of CODH/ACS isoforms present in M. acetivorans.
Function of the Cdh isoforms in M. acetivorans.
The genome of M. acetivorans C2A encodes, like those of Methanosarcina barkeri and M. mazei, two isoforms of CODH/ACS in putative transcriptional units. Unique to M. acetivorans is the presence of a single cdhA3 gene. Previously, analysis of CODH/ACS-encoding transcripts in M. mazei, a close relative of M. acetivorans, led to the proposal that the two isoforms may play distinct metabolic roles, one more active in synthesizing acetyl-CoA for biosynthetic purposes and the other more active in cleaving acetyl-CoA during energy metabolism (11). The genome of M. thermophila encodes only one CODH/ACS that has to be active in both directions, acetyl-CoA synthesis and cleavage (18). Here, we provide unambiguous experimental evidence that each Cdh isoform of M. acetivorans is capable of both catabolic and anabolic functions, i.e., that both Cdh isoforms are bona fide CODH/ACS, by showing that (i) one isoform is required and sufficient for aceticlastic and carboxidotrophic growth and that (ii) lack of both isoforms renders the respective mutant auxotrophic for acetate. Still, both the mutational and gene expression analyses argue that both isoforms do not share equal functionality but that Cdh2 is the major isoform because it is expressed at a higher level than Cdh1 under all tested conditions, and consequently, its loss results in more severe growth defects. It therefore appears that in M. acetivorans, Cdh1 plays an auxiliary role that is beneficial under certain physiological conditions.
Regulation of the CODH/ACS system in M. acetivorans.
We confirmed for M. acetivorans the findings of previous analyses, which showed that transcription of CODH/ACS-encoding genes is strongly regulated by the growth substrate. The resulting expression levels are consistent with the enzyme's catabolic role during aceticlastic growth and its anabolic role during methylotrophic growth (2, 45). The cdhABCDE transcript of M. thermophila contains a 371-nucleotide 5′ untranslated (UTR) leader sequence, which is involved in regulating transcription elongation into the cdhA coding region in a growth substrate-dependent fashion (1). The corresponding sequence of the putative cdhA2 5′ UTR in M. acetivorans is very similar to the one in M. thermophila and could, therefore, be one means of regulating transcription in M. acetivorans. Noteworthy, the reporter gene fusion constructs used here contain all transcription signals, including the promoters on the DNA and the resulting 5′ UTR on the mRNA. The observation that expression of the cdhA genes is significantly higher during growth on acetate than during growth on CO may be partly explained by the different amounts of carbon channeled through CODH/ACS for energy conservation and biosynthesis. During growth of M. acetivorans on CO, 8 to 14% of the carbon is metabolized via acetyl-CoA, and thus CODH/ACS, for acetate and cell mass formation (37). During growth of Methanosarcina species on acetate, 98 to 99% of the carbon is metabolized to methane and CO2 via CODH/ACS (42, 47). Also, very little free energy is available from aceticlastic methanogenesis, necessitating a high substrate turnover, and thus high levels of CODH/ACS, to conserve enough energy for growth (10).
Although expression levels of cdhA1 and cdhA2 in the wild type are at least 4-fold lower during growth on CO than during growth on acetate, the overall CODH activity differs only 2-fold at most between the two substrates, which implies growth substrate-dependent regulation of the CODH/ACS system beyond that of transcription. Carboxidotrophic methanogenesis in M. acetivorans is relatively slow (33), and regulating CODH/ACS not only on the transcriptional level may be advantageous for the organism. To achieve this, CODH/ACS activity or the half-lives of the enzymes could be directly affected by cellular effectors, like the substrates or products, other small molecules, or other enzymes. Such signals could also affect transcript stability or efficiency of translation. However, nothing is known about posttranscriptional gene regulation in methanogens. Still, this level of regulation is obviously also influenced by the genetic context. For example, the acetate-dependent growth defect observed in the cdh2 mutant (MCD2) is fully compensated, probably by Cdh1, after prolonged cultivation on this substrate. In contrast to what was expected, cdh1 expression in this strain was not increased but decreased, which suggests posttranscriptional processes leading to increased CODH/ACS activity responsible for this compensation. However, pleiotropic mutations at some second site(s) that improve the fitness of the mutants on acetate cannot be ruled out.
Our analysis further indicates that there are genetic interactions between the cdh coding loci or direct interactions between the proteins themselves. The fact that CODH activities of the cdh1 and cdh2 mutants combined are greater than that of the wild type under any conditions tested strongly suggests a regulatory cross talk within the CODH/ACS system, directly effecting either enzyme activity or gene expression. The fact that absence of Cdh2 results in an approximately 50% decrease of cdhA1 expression during growth on CO or acetate argues for the latter scenario. However, how this effect could be brought about cannot be plausibly explained at present, but such changes in expression of a gene upon disruption of isogenes have been observed in other organisms (21, 40).
CdhA3 seems to be involved in regulation of the CODH/ACS system of M. acetivorans.
This study further revealed that CdhA3 affects, to various degrees and in different directions, regulation of the CODH/ACS system on both the transcriptional and posttranscriptional levels. Although we were not able to determine expression of cdhA3 using reporter gene fusions, microarray analyses showed that cdhA3 is expressed (27). While deletion of cdhA3 leads to a slight decrease of cdhA2 expression, transcription of cdhA1 is significantly increased, but only under aceticlastic conditions. Under carboxidotrophic conditions, cdhA2 expression is increased when CdhA3 is absent, which may explain the improved growth under this condition. While the data presented clearly indicate that CdhA3 affects transcription, it is very unlikely that CdhA3 directly acts as a regulator of cdh gene expression, because CdhA3 harbors no motif suggestive of binding to DNA or RNA and no other CODH is known to interact with nucleic acids. Instead, CdhA3 could be part of a catabolite responsive signal transduction pathway. The proteobacterium Ralstonia eutropha (also termed Wautersia eutropha and Cupriavidus necator) contains, beside two catabolic hydrogenases, a regulatory hydrogenase (HoxBC) forming a complex with the histidine kinase HoxJ, thereby relaying H2 status to the response regulator HoxA, which activates expression of hydrogenase genes (25). Methanogenic CODH/ACS multienzyme complexes exchange the carbonyl of acetyl-CoA with exogenous CO (36). It is therefore feasible that CdhA3 may sense CO released during acetyl-CoA cleavage, thus integrating both CO and acetate as metabolic signals. Interestingly, the ORFs (ma1017 and ma3866, respectively) upstream of cdhA1 (ma1016) and downstream of cdhE2 (ma3865) encode putative helix-turn-helix motif-containing proteins, often characterized by binding of nucleic acid, which could, therefore, be involved in transcriptional regulation of the CODH/ACS system of M. acetivorans. Despite the fact that M. acetivorans does not encode homologs of CooA or RcoM (23, 41), the only CO sensors known thus far, M. acetivorans is the most CO tolerant among the methanogens investigated in this respect (32) and specifically alters its protein inventory in response to CO (26, 39). Probably not coincidentally, it is also the only Methanosarcina species (with a published genome sequence) encoding CdhA3 (see Fig. S2 in the supplemental material). M. acetivorans, thus, appears to have evolved a unique system to sense CO, to acclimate to this toxic gas, and to regulate the pathways of its utilization.
Beside this very hypothetical role in a potential signal transduction pathway it is also feasible that CdhA3 may affect CODH/ACS more directly. The fact that CODH activity during growth on acetate of the cdh2 cdhA3 double mutant (MCD32) is almost 3-fold higher than that of the wild type and more than 5-fold higher than that of the cdh2 mutant suggests that CdhA3 may influence the enzyme's activity, for example by direct binding or by binding of an effector. Clearly, further investigations toward the role of CdhA3 are warranted to resolve this interesting issue, and the cdh mutants are uniquely suited for these analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kerstin Yacoub for excellent technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to M.R. (SPP 1112 and RO 2445/6-1).
Footnotes
Published ahead of print 3 August 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Anderson KL, Apolinario EE, MacAuley SR, Sowers KR. 2009. A 5′ leader sequence regulates expression of methanosarcinal CO dehydrogenase/acetyl coenzyme A synthase. J. Bacteriol. 191:7123–7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apolinario EE, Jackson KM, Sowers KR. 2005. Development of a plasmid-mediated reporter system for in vivo monitoring of gene expression in the archaeon Methanosarcina acetivorans. Appl. Environ. Microbiol. 71:4914–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ausubel FM, et al. 2003. Current protocols in molecular biology. J. Wiley & Sons, Inc., New York, NY [Google Scholar]
- 4. Boccazzi P, Zhang JK, Metcalf WW. 2000. Generation of dominant selectable markers for resistance to pseudomonic acid by cloning and mutagenesis of the ileS gene from the archaeon Methanosarcina barkeri Fusaro. J. Bacteriol. 182:2611–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bose A, Metcalf WW. 2008. Distinct regulators control the expression of methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. Mol. Microbiol. 67:649–661 [DOI] [PubMed] [Google Scholar]
- 6. Bose A, Pritchett MA, Rother M, Metcalf WW. 2006. Differential regulation of the three methanol methyltransferase isozymes in Methanosarcina acetivorans C2A. J. Bacteriol. 188:7274–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 8. Deppenmeier U, et al. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between bacteria and archaea. J. Mol. Microbiol. Biotechnol. 4:453–461 [PubMed] [Google Scholar]
- 9. Deppenmeier U, Lienard T, Gottschalk G. 1999. Novel reactions involved in energy conservation by methanogenic archaea. FEBS Lett. 457:291–297 [DOI] [PubMed] [Google Scholar]
- 10. Deppenmeier U, Müller V. 2008. Life close to the thermodynamic limit: how methanogenic archaea conserve energy, p 123–152 In Schäfer G, Penefsky HS. (ed), Bioenergetics: energy conservation and conversion, vol 45 Springer, Heidelberg, Germany: [DOI] [PubMed] [Google Scholar]
- 11. Eggen RI, et al. 1996. Carbon monoxide dehydrogenase from Methanosarcina frisia Gö1: characterization of the enzyme and the regulated expression of two operon-like cdh gene clusters. J. Biol. Chem. 271:14256–14263 [DOI] [PubMed] [Google Scholar]
- 12. Ferry JG. 1997. Enzymology of the fermentation of acetate to methane by Methanosarcina thermophila. BioFactors 6:25–35 [DOI] [PubMed] [Google Scholar]
- 13. Ferry JG. 1992. Methane from acetate. J. Bacteriol. 174:5489–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiedler S, Wirth R. 1988. Transformation of bacteria with plasmid DNA by electroporation. Anal. Biochem. 170:38–44 [DOI] [PubMed] [Google Scholar]
- 15. Galagan JE, et al. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grahame DA. 1991. Catalysis of acetyl-CoA cleavage and tetrahydrosarcinapterin methylation by a carbon monoxide dehydrogenase-corrinoid enzyme complex. J. Biol. Chem. 266:22227–22233 [PubMed] [Google Scholar]
- 17. Grahame DA, DeMoll E. 1996. Partial reactions catalyzed by protein components of the acetyl-CoA decarbonylase synthase enzyme complex from Methanosarcina barkeri. J. Biol. Chem. 271:8352–8358 [DOI] [PubMed] [Google Scholar]
- 18. Grahame DA, Gencic S, DeMoll E. 2005. A single operon-encoded form of the acetyl-CoA decarbonylase/synthase multienzyme complex responsible for synthesis and cleavage of acetyl-CoA in Methanosarcina thermophila. Arch. Microbiol. 184:32–40 [DOI] [PubMed] [Google Scholar]
- 19. Guss AM, Mukhopadhyay B, Zhang JK, Metcalf WW. 2005. Genetic analysis of mch mutants in two Methanosarcina species demonstrates multiple roles for the methanopterin-dependent C-1 oxidation/reduction pathway and differences in H2 metabolism between closely related species. Mol. Microbiol. 55:1671–1680 [DOI] [PubMed] [Google Scholar]
- 20. Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183:6384–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holtmann G, Bakker EP, Uozumi N, Bremer E. 2003. KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 185:1289–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerby RL, Ludden PW, Roberts GP. 1995. Carbon monoxide-dependent growth of Rhodospirillum rubrum. J. Bacteriol. 177:2241–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kerby RL, Youn H, Roberts GP. 2008. RcoM: a new single-component transcriptional regulator of CO metabolism in bacteria. J. Bacteriol. 190:3336–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ladapo J, Whitman WB. 1990. Method for isolation of auxotrophs in the methanogenic archaebacteria: Role of the acetyl-CoA pathway of autotrophic CO2 fixation in Methanococcus maripaludis. Proc. Natl. Acad. Sci. U. S. A. 87:5598–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lenz O, Bernhard M, Buhrke T, Schwartz E, Friedrich B. 2002. The hydrogen-sensing apparatus in Ralstonia eutropha. J. Mol. Microbiol. Biotechnol. 4:255–262 [PubMed] [Google Scholar]
- 26. Lessner DJ, et al. 2006. An unconventional pathway for reduction of CO2 to methane in CO-grown Methanosarcina acetivorans revealed by proteomics. Proc. Natl. Acad. Sci. U. S. A. 103:17921–17926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, et al. 2007. Quantitative proteomic and microarray analysis of the archaeon Methanosarcina acetivorans grown with acetate versus methanol. J. Proteome Res. 6:759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maeder DL, et al. 2006. The Methanosarcina barkeri genome: comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J. Bacteriol. 188:7922–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Metcalf WW, Zhang JK, Apolinario E, Sowers KR, Wolfe RS. 1997. A genetic system for Archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. U. S. A. 94:2626–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metcalf WW, Zhang JK, Shi X, Wolfe RS. 1996. Molecular, genetic, and biochemical characterization of the serC gene of Methanosarcina barkeri Fusaro. J. Bacteriol. 178:5797–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran JJ, House CH, Vrentas JM, Freeman KH. 2008. Methyl sulfide production by a novel carbon monoxide metabolism in Methanosarcina acetivorans. Appl. Environ. Microbiol. 74:540–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oelgeschläger E, Rother M. 2008. Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch. Microbiol. 190:257–269 [DOI] [PubMed] [Google Scholar]
- 33. Oelgeschläger E, Rother M. 2009. Influence of carbon monoxide on metabolite formation in Methanosarcina acetivorans. FEMS Microbiol. Lett. 292:254–260 [DOI] [PubMed] [Google Scholar]
- 34. Pritchett MA, Zhang JK, Metcalf WW. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl. Environ. Microbiol. 70:1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rasche ME, Terlesky KC, Abbanat DR, Ferry JG. 1995. Purification of carbon monoxide dehydrogenase from Methanosarcina thermophila, p 231–235 In Robb FT, Place ER, Sowers KR, Schreier HJ. (ed), Archaea—a laboratory manual, vol 3 Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 36. Raybuck SA, et al. 1991. Demonstration of carbon-carbon bond cleavage of acetyl coenzyme A by using isotopic exchange catalyzed by the CO dehydrogenase complex from acetate-grown Methanosarcina thermophila. J. Bacteriol. 173:929–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rother M, Metcalf WW. 2004. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. U. S. A. 101:16929–16934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rother M, Metcalf WW. 2005. Genetic technologies for Archaea. Curr. Opin. Microbiol. 8:745–751 [DOI] [PubMed] [Google Scholar]
- 39. Rother M, Oelgeschläger E, Metcalf WW. 2007. Genetic and proteomic analyses of CO utilization by Methanosarcina acetivorans. Arch. Microbiol. 188:463–472 [DOI] [PubMed] [Google Scholar]
- 40. Saum R, Mingote A, Santos H, Müller V. 2009. Genetic analysis of the role of the ABC transporter Ota and Otb in glycine betaine transport in Methanosarcina mazei Gö1. Arch. Microbiol. 191:291–301 [DOI] [PubMed] [Google Scholar]
- 41. Shelver D, Kerby RL, He Y, Roberts GP. 1995. Carbon monoxide-induced activation of gene expression in Rhodospirillum rubrum requires the product of cooA, a member of the cyclic AMP receptor protein family of transcriptional regulators. J. Bacteriol. 177:2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith MR, Mah RA. 1980. Acetate as sole carbon and energy source for growth of Methanosarcina strain 227. Appl. Environ. Microbiol. 39:993–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sowers KR, Baron SF, Ferry JG. 1984. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sowers KR, Boone JE, Gunsalus RP. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 59:3832–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sowers KR, Thai TT, Gunsalus RP. 1993. Transcriptional regulation of the carbon monoxide dehydrogenase gene (cdhA) in Methanosarcina thermophila. J. Biol. Chem. 268:23172–32178 [PubMed] [Google Scholar]
- 46. Thauer RK. 1998. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiology 144:2377–2406 [DOI] [PubMed] [Google Scholar]
- 47. Touzel JP, Petroff D, Albagnac G. 1985. Isolation and characterization of a new thermophilic Methanosarcina, the strain CHTI 55. Syst. Appl. Microbiol. 6:66–71 [Google Scholar]
- 48. Wanner BL. 1986. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J. Mol. Biol. 191:39–58 [DOI] [PubMed] [Google Scholar]
- 49. Welander PV, Metcalf WW. 2008. Mutagenesis of the C1 oxidation pathway in Methanosarcina barkeri: new insights into the Mtr/Mer bypass pathway. J. Bacteriol. 190:1928–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.