Abstract
Previously, it has been shown that infection in humans with the pandemic swine influenza virus induces antibodies with specificity to the stalk domain of the viral hemagglutinin. Following the generation of these data, we sought to recapitulate these findings in the mouse model by sequential influenza virus infection. Mice that were inoculated with a seasonal influenza H1N1 virus followed by infection with a pandemic H1N1 strain produced higher antihemagglutinin stalk antibody titers than mice sequentially infected with drifted seasonal strains. In order to achieve antibody titers of comparable magnitude using sequential infection, mice had to be infected with 100- to 1,000-fold more of the drifted seasonal virus. The antistalk antibodies produced by these infections were influenza virus neutralizing, which illustrates the utility of the mouse model in which to study this interaction between virus and host.
INTRODUCTION
The hemagglutinin (HA) of influenza virus is a large surface glycoprotein that modulates attachment and entry of the virus to host cells. Once the virus is endocytosed and enters cells, a conformational change is induced in the HA that allows for the fusion of viral and endosomal membranes and the release of the viral genome into the cytoplasm for its subsequent import to the nucleus. Based on these functions, the protein can be divided into two parts: (i) a globular head domain that harbors the receptor binding pocket, as well as immunodominant antigenic sites, and (ii) the membrane-proximal stalk domain, where the fusion peptide is located (11).
Several well-defined antigenic sites surround the receptor binding pocket in the head domain of the HA. Antibodies directed against these sites can block influenza virus entry and binding to host cells, and as such, these antibodies have hemagglutination-inhibiting (HI) activity. Due to the high variability of the HA head across influenza virus subtypes, these antibodies are usually only specific to the virus against which they were raised and have little cross-reactivity between different influenza virus strains. The selective pressure placed upon the HA head by these antibodies drives the accumulation of point mutations to evade the host immune response, a process known as antigenic drift, and contributes to a substantial part of the diversity seen across influenza virus subtypes and even between strains within subtypes. Current strategies of influenza virus vaccination aim to elicit these types of antibodies, with the quantification of serum HI titer as a correlate of protection (11).
A recently much discussed and rediscovered class of neutralizing antibodies has been shown to target the conserved stalk domain of the hemagglutinin. These antibodies are thought to hinder the conformational change of the HA following entry, thereby inhibiting fusion of the viral membrane with the endosomal membrane of the host (1–3, 12, 16, 18). Because the stalk domain of the HA is highly conserved across influenza virus subtypes, unlike the globular head domain, these antibodies have been known to be reactive to a variety of influenza virus strains (1–3, 8, 10, 16, 18, 20).
Interestingly, it has been demonstrated that monoclonal antibodies isolated from individuals infected with the 2009 pandemic virus were affinity matured and reactive to influenza viruses of various subtypes, with many of these antibodies directed against the HA stalk (21). Using techniques that directly measure the quantity of stalk-specific antibodies in polyclonal serum samples, it was shown that patients infected with the 2009 pandemic virus had higher titers of stalk-specific antibodies than uninfected controls (13). Based on these findings, it has been hypothesized that exposure to a virus with an antigenically novel HA selectively recalled and boosted memory B cells toward portions of the HA that are well conserved between pandemic and seasonal strains, such as epitopes within the stalk domain, and that these antibodies on a population level caused the replacement of seasonal H1N1 strains with those that circulate at present (12, 13).
Here we attempt to study this phenomenon in more detail using the mouse model by sequentially infecting mice with seasonal and pandemic H1N1 influenza viruses and assessing the degree to which antistalk antibodies were generated using previously described tools, chimeric HA (cHA) proteins and pseudotyped virus particles expressing cHAs (6, 13). We demonstrate that infection with a seasonal influenza virus followed by infection with a pandemic strain stimulated the production of stalk-specific antibodies in mice. While antibodies with specificity to the HA stalk could also be produced from infection with only drifted seasonal isolates, substantially more virus was needed to produced comparable stalk antibody titers. Our findings mirror what has been seen in humans and validate the mouse model for the further exploration of this phenomenon.
MATERIALS AND METHODS
Cells and viruses.
293T and MDCK cells were obtained from ATCC and were maintained in Dulbecco's modified Eagle's medium (DMEM) and minimal essential medium (both from Gibco), respectively, each supplemented with 10% fetal calf serum (HyClone) and 100 U/ml of penicillin–100 μg/ml of streptomycin (Pen/Strep) (Gibco).
Influenza virus strains A/New Caledonia/20/99 (NC99) (H1N1), A/Solomon Islands/3/2006 (SI06) (H1N1), A/Puerto Rico/8/1934 (PR8) (H1N1), A/Fort Monmouth/1/1947 (FM1) (H1N1), and A/California/04/2009 (Cal09) (H1N1) and a low-pathogenicity A/Vietnam/1203/04 (VN04):PR8 2:6 reassortant virus (H5N1) (14) were grown in 10-day-old embryonated eggs for 48 h.
In order to construct a cold-adapted virus with PR8 antigenicity, an A/Ann Arbor/6/60-based rescue system was generated by reverse transcription of viral genes (Transcriptor reverse transcriptase; Roche) from purified virion RNA, PCR amplification (PFU Turbo; Stratagene), and cloning into the vector pPOL1 (5) following the recombinational protocol described by Wang et al. (19). A/Ann Arbor/6/60 plasmids encoding the polymerase basic 1 (PB1) and PB2 proteins, polymerase acidic (PA) protein, nucleoprotein (NP), and matrix (M) and nonstructural (NS) proteins were used with those encoding HA and neuraminidase (NA) from the PR8 strain to rescue a PR8-based cold-adapted virus. The cold-adapted virus was grown in 10-day-old embryonated chicken eggs for 48 h. In order to construct a PR8 virus with a deletion in nonstructural protein 1 (NS1), a plasmid that only encodes the first 73 amino acids of NS1 was used in addition to the seven other PR8-based rescue plasmids. Following its rescue, the virus was propagated in 8-day-old embryonated chicken eggs for 48 h (9).
The titers of recombinant and wild-type viruses were determined on MDCK cells (ATCC) in the presence of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK) trypsin as previously described (6). Viruses were inactivated following formaldehyde treatment for 72 h at 4°C.
Recombinant baculovirus generation, protein expression, and purification.
In order to generate NC99, Cal09, cH6/1 (the globular head domain from H6N1 A/mallard/Sweden/81/02 and the stalk domain from PR8), or VN04 HA and VN04 NA, a baculovirus-based expression system was employed as previously described (13). Briefly, baculotransfer vectors were transformed into Escherichia coli strain DH10Bac (Invitrogen), and colonies were picked and grown up. Bacmids were prepared using a plasmid midikit (Qiagen) and then transfected into Sf9 cells with Cellfectin II (Invitrogen) according to the manufacturer's instructions. Recombinant baculovirus was amplified in Sf9 cells grown in TNM-FH medium (Gemini Bioproducts) and then used to infect High Five cells grown in HyClone SFX insect cell medium (Thermo Fisher Scientific) at a multiplicity of infection (MOI) of 10. Supernatants were harvested 96 h postinfection and then incubated with Ni-nitrilotriacetic acid (NTA) resin (Qiagen) for 2 h at 4°C to purify His-tagged recombinant HA proteins. The slurry was loaded onto columns and washed three times with washing buffer (50 mM Na2HCO3, 300 mM NaCl, 20 mM imidazole [pH 8]). Protein was eluted in 0.5-ml steps with elution buffer (50 mM Na2HCO3, 300 mM NaCl, 250 mM imidazole [pH 8]) and tested for protein content with Bradford reagent, and fractions containing protein were pooled. Pooled fractions were buffer exchanged in phosphate-buffered saline (PBS) and concentrated using an Amicon Ultra centrifugal filter unit (Millipore) with a 10-kDa molecular mass cutoff in a swinging bucket rotor. Protein purity and identity were tested by SDS-PAGE, Coomassie staining, and Western blotting. Final protein concentrations were determined with Bradford reagent.
Animals.
All animal experiments were performed in accordance with the guidelines of the Mount Sinai School of Medicine Institutional Animal Care and Use Committee. Animals were allowed access to food and water ad libitum and kept on a 12-h light/dark cycle. Female 6- to 8-week-old BALB/c mice (Jackson Laboratories) were anesthetized for all intranasal procedures with intraperitoneal (i.p.) injection of 0.1 ml of ketamine-xylazine (0.15 mg ketamine and 0.03 mg xylazine).
Infection and vaccination.
Groups of five 6- to 8-week-old female BALB/c mice were anesthetized and inoculated intranasally with 50 μl of 104 PFU of either NC99, SI06, or Cal09 viruses, 103 or 104 PFU of cold-adapted PR8 virus, or 103 or 104 PFU of an NS1-truncated PR8 virus diluted in PBS. Mice also received 1 μg of formaldehyde-inactivated PR8 or FM1 virus or the commercial trivalent split vaccine containing 1 μg of A/Brisbane/57/07 (TIV) H1 HA (the dose also contained 1 μg of the H3 component A/Uruguay/716/07 and the B component B/Brisbane/60/08) administered intramuscularly in a volume of 50 μl. Additionally, two groups of mice were vaccinated with 80 μg of pCAGGS plasmid encoding either PR8 or NC99 HA using a TriGrid electroporation device (Ichor Medical Systems) (15). Four weeks following infection or vaccination, animals were bled retro-orbitally and serum was harvested from whole blood.
Following infection with NC99 virus, mice were intranasally inoculated with 104, 105, or 106 PFU of SI06 virus or with 103 or 104 PFU of Cal09 virus diluted in PBS. Three days postboost, three animals per group were euthanized with CO2 and lungs were harvested and homogenized with a FastPrep-24 homogenizer (MP). Lung virus titers were measured by titration on MDCK cells. Four weeks following the second infection, animals were bled retro-orbitally and serum was harvested from whole blood.
ELISA.
Immulon 4HBX (Thermo Scientific) plates were coated overnight with purified baculovirus expressed NC99, Cal09, cH6/1, or VN04 HA (all with a C-terminal T4 foldon) or VN04 NA (with an N-terminal tetramerization domain) in coating buffer (0.1 M Na2CO3-NaHCO3 [pH 9.2], 50 μl/well) or PBS (13). Plates were blocked for 1 h with 0.1% Tween 20-PBS (TPBS) containing 3% nonfat milk powder and then incubated with mouse serum serially diluted in TPBS containing 1% milk powder for 1 h at room temperature. After three washes, plates were incubated for 1 h at room temperature with a horseradish-peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (Sigma) or an alkaline phosphate (AP)-linked anti-mouse IgG (γ-chain specific; Invitrogen). Plates were then washed three times with TPBS and developed. When the HRP-conjugated secondary antibody was used, plates were developed using SigmaFAST o-phenylenediamine dihydrochloride (OPD) substrate (Sigma) (100 μl/well), stopped with 3 M HCl, and read at 490 nm. When an AP-linked secondary antibody was used, plates were developed with p-nitrophenylphosphate (PNPP) substrate (Zymed), stopped with 0.5 M NaOH, and read at the optical density at 405 nm (OD405). For all experiments, a Synergy 4 (BioTek) plate reader was used. For enzyme-linked immunosorbent assay (ELISA) experiments detecting reactivity to H5 HA, sera from mice infected with 105 PFU of an NS1-truncated A/Vietnam/1203/2004 virus were used as a positive control.
Purification of mouse IgG from polyclonal sera.
Mouse serum was diluted in PBS (pH 7.4) and passed through a 0.45-μm sterile filter unit. Filtered sera were loaded on a column containing 3 ml of 4 FastFlow Sepharose G (GE Healthcare). The column was washed with 60 ml of PBS, and total IgG was eluted with a 0.1 M glycine HCl buffer (pH 2.7) and immediately neutralized using a 2 M Tris-HCl buffer (pH 10) (7). Eluted IgG was then concentrated and buffer exchanged (to PBS) using Amicon Ultracell (Millipore) centrifugation units with a cutoff of 30 kDa. The protein concentration was measured on a NanoDrop 2000 spectrophotometer using the A280 method.
Pseudotyped particle neutralization assay.
The procedure for pseudotype particle production was adapted from previous studies and has been previously described (4, 6, 13). Briefly, 293T cells were cotransfected with four plasmids encoding a provirus containing a luciferase reporter gene, HIV Gag-Pol, the chimeric cH5/1 hemagglutinin protein (A/Viet Nam/1203/04 H5 head domain and PR8 stalk domain), and a neuraminidase from influenza B virus B/Yamagata/16/88. Supernatants were collected 48 h posttransfection and subsequently filtered (0.45-μm-pore size) in order to purify the cH5/1 particle preparations. Particles were then incubated with different concentrations of purified mouse IgGs and added to MDCK cells. Transductions proceeded for 6 h before cells were washed, and fresh medium was placed over cells. All transductions were performed in the presence of 1 μg/ml Polybrene (Sigma, St. Louis, MO). Luciferase assays were performed 48 h after transduction.
Passive transfer experiment.
Mice (n = 5 per group) were intraperitoneally inoculated with 200 μl of sera from groups that were infected with NC99 virus, NC99 followed by Cal09 or SI06 virus, or sera from naïve animals. Two hours postinoculation, mice were anesthetized and challenged with 5 50% minimal lethal doses (MLD50) of the VN04:PR8 2:6 reassortant (14). Weight loss was monitored daily for 14 days, and mice that lost more than 30% of their initial body weight were scored as dead and euthanized. Statistical analyses were performed using Prism4 (GraphPad). Differences in survival were calculated by Kaplan-Meier survival analysis with log rank significance test. P values of ≤0.05 are considered statistically significant.
RESULTS
Stalk-reactive antibodies are induced upon infection with seasonal or pandemic H1N1 virus but not by vaccination.
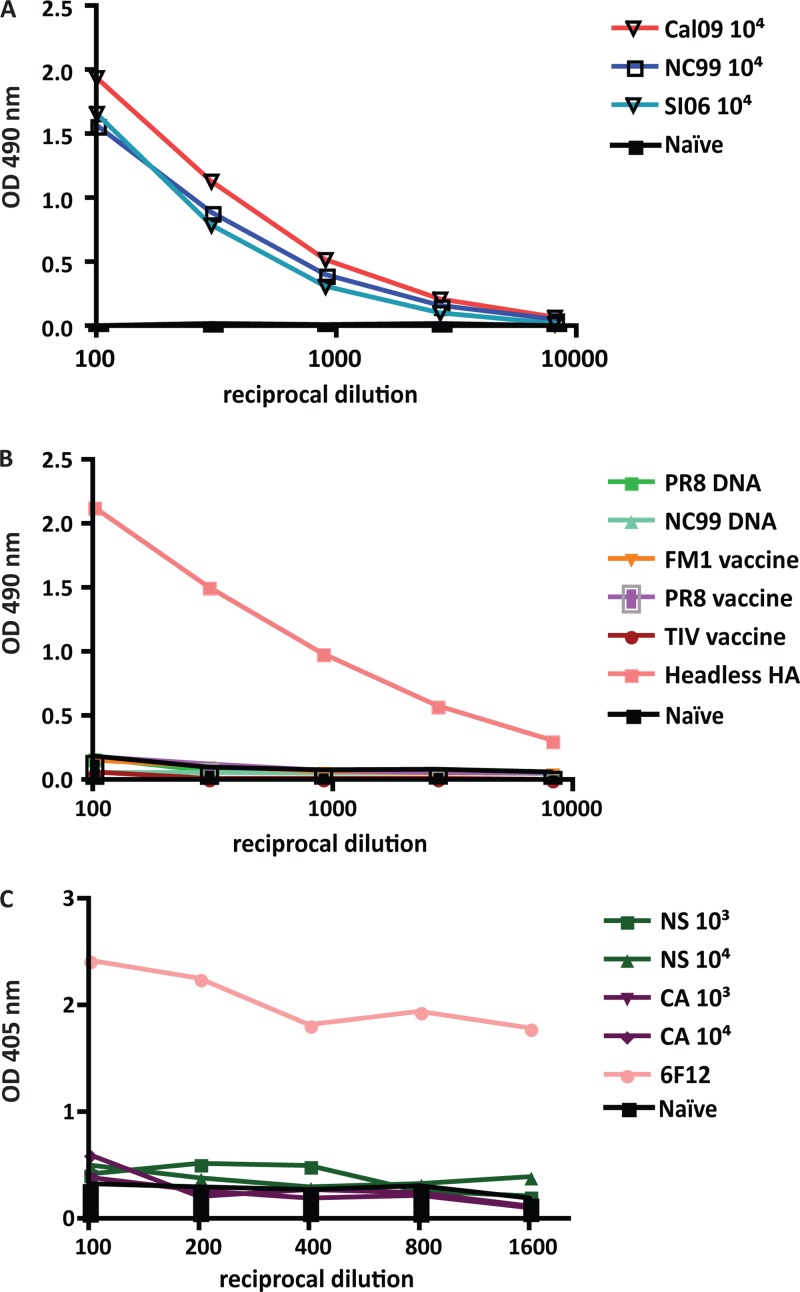
Groups of five mice were sublethally infected with 104 PFU of NC99, SI06, or Cal09 virus and bled 4 weeks later for the assessment of serum stalk antibody titer. In order to determine the degree of stalk antibodies induced by infection, cH6/1 protein, a soluble HA construct that contains the stalk of an H1 virus and the head of an H6 virus, was used (13). This reagent allows for the direct detection of stalk-specific antibodies in polyclonal sera. All mice infected with a seasonal or pandemic virus produced antibodies with reactivity to the HA stalk (Fig. 1A).
Fig 1.
Hemagglutinin stalk antibodies are produced following replicative infection. Animals were infected with 104 PFU of Cal09, NC99, or SI06 virus (A), vaccinated with PR8 DNA, NC99 DNA, FM1 inactivated virus, or PR8 inactivated virus (B), or vaccinated with 103 or 104 PFU live attenuated cold-adapted or NS1-truncated viruses expressing PR8 HA (C). Sera were harvested from mice 4 weeks after infection or vaccination, and hemagglutinin stalk-specific antibodies were assayed by ELISA using cH6/1 protein. Serum raised from mice vaccinated with headless hemagglutinin and monoclonal antibody 6F12 was used as a positive control.
In contrast, animals that were electroporated with DNA encoding PR8 or NC99 HA or those that received inactivated PR8 or FM1 vaccine or the commercial split vaccine intramuscularly did not have any reactivity to cH6/1 by ELISA (Fig. 1B), despite their seroconversion to the antigen with which they were vaccinated (see Fig. S1 in the supplemental material). When animals were vaccinated with 103 or 104 of a cold-adapted or live virus attenuated via NS1 truncation, stalk-specific antibody titers were barely above the background level (Fig. 1C). Sera from mice vaccinated with a headless hemagglutinin construct (15) and stalk-specific monoclonal antibody 6F12 were used as positive controls (17). Since the replication level of the attenuated viruses is lower than that of wild-type viruses and inactivated/DNA vaccines did not induce stalk-reactive antibodies, we hypothesize that the initial induction of these antibodies is greatly enhanced by replication-competent virus.
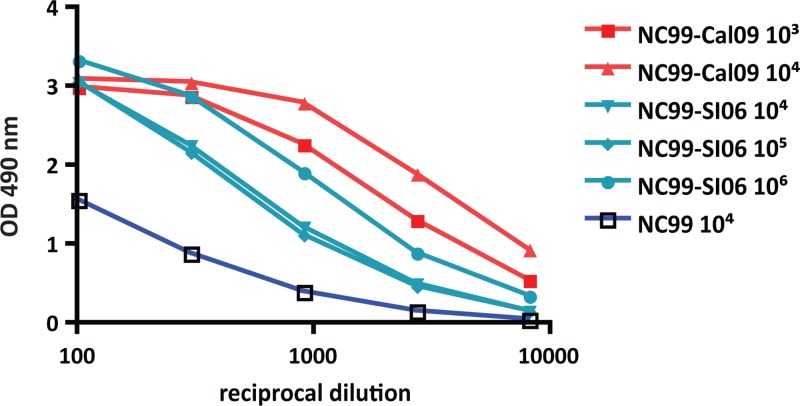
A boost with pandemic H1N1 elicits higher titers of stalk-reactive antibodies than with a drifted seasonal H1N1 isolate.
Animals primed with 104 PFU of NC99 virus were boosted 4 weeks postpriming with two different doses of SI06 (105 and 106 PFU [NC99-SI06]) or Cal09 (103 and 104 PFU [NC99-Cal09]). Four weeks later, mice were terminally bled and sera were analyzed for the presence of stalk-reactive antibodies using recombinant cH6/1 protein. All animals that received a second inoculation of virus had elevated stalk titers compared to mice that had only been infected with NC99 (Fig. 2). Indeed, the effect was dose dependent, as animals that received the lower dose (103 PFU of Cal09 or 105 PFU of SI06 virus) had a weaker boost than those that received a higher dose (104 PFU of Cal09 or 106 PFU of SI06 virus). It is of note, however, that NC99-Cal09-infected animals exhibited a stronger induction of stalk titer than the NC99-SI06-infected group. In fact, in order to generate comparable serum stalk titers to the NC99-Cal09 group, 1,000-fold more of the seasonal virus SI06 was required (Fig. 2). In order to confirm seroconversion to primary and secondary infections, all sera were tested for reactivity to recombinant NC99 and Cal09 HA protein by ELISA (see Fig. S1 in the supplemental material).
Fig 2.
Hemagglutinin stalk antibodies are boosted following a second exposure to influenza virus. Animals were infected with 104 PFU of NC99 and then boosted 4 weeks later with 104, 105, or 106 PFU of SI06 virus or 103 or 104 PFU of Cal09 virus. Sera were harvested from mice 4 weeks after the second infection, and hemagglutinin stalk-specific antibodies were assayed by ELISA using cH6/1 protein. Values shown in Fig. 1 for animals that only received one inoculation of NC99 virus were included to serve as a comparison.
Based on the finding that replication strongly enhances the elicitation of stalk-directed antibodies, we rationalized that the increased boost in antistalk antibody titer seen in Cal09-infected animals could be a result of enhanced replication capability of the virus. Three days postboost, NC99-Cal09 and NC99-SI06-infected animals were sacrificed and lung tissues were collected. While we were unable to detect SI06 virus in the lungs of sequentially infected mice, Cal09 virus grew to 105 PFU/ml by this time point (Table 1).
Table 1.
Day 3 postboost lung titers
| Boost (PFU)a | Viral lung titer (PFU/ml)b |
|---|---|
| Cal09 | |
| 103 | 1.15 × 105 |
| 104 | 2.44 × 105 |
| SI06 | |
| 104 | NDc |
| 105 | ND |
| 106 | ND |
Following priming with 104 PFU A/New Caledonia/20/99 (NC99) virus, animals were boosted with various doses of A/California/04/09 (Cal09) or A/Solomon Islands/3/06 (SI06) virus.
Three days postboost, lungs were harvested and viral titers were determined by plaque assay.
ND, not detected.
Elicited stalk-reactive antibodies have neutralizing activity in vitro.
In order to assess the neutralizing capability of these stalk-specific antibodies, a pseudotyped particle neutralization assay was used. Pseudoparticles that express an HA with an H1 stem and an H5 head (cH5/1) were engineered to harbor a luciferase reporter gene that would be expressed following the successful entry of the pseudoparticle into cells. Entry is therefore measured as a function of luciferase expression. Because animals were only exposed to H1N1 viruses, we would then argue that any inhibition of entry would be a result of neutralizing antibodies directed toward the HA (H1) stalk. Due to the limited amount of serum available, we decided to test the relative neutralization efficiency of purified IgG preparations of serum from mice that were primed with NC99 virus but then were boosted with 103 PFU Cal09 virus or 105 PFU SI06 virus. Serum from mice that were infected with Cal09 alone was used as an additional point of comparison. Monoclonal antibody 6F12 (17), a stalk-reactive antibody with broad group 1 specificity, was used as a positive control.
Greater than 90% inhibition was seen with as little as 50 μg/ml of purified IgG from either NC99-Cal09- or NC99-SI06-infected mice (Table 2). It is of note that IgGs isolated from NC99-Cal09-infected mice and NC99-SI06-infected mice were able to inhibit the entry of cH5/1-expressing pseudoparticles with similar efficiencies at 50 or 10 μg/ml. IgG from mice infected with only Cal09 virus displayed low levels of entry inhibition, which was similar to the levels seen for naïve mice. Again, this reflects the relative stalk antibody titers in this group, as shown in Fig. 2.
Table 2.
Percentage of inhibition of cH5/1-pseudotyped particle entry using a purified IgG preparation from pooled sera
| IgG concn (μg/ml) | % inhibition with: |
||||
|---|---|---|---|---|---|
| NC99-Cal09a | NC99-SI06b | Cal09c | 6F12d | Naïvee | |
| 50 | 92.0 ± 4.2 | 97.3 ± 0.9 | 9.0 ± 12.8 | 99.8 ± 0.0 | 0 ± 0 |
| 10 | 50.5 ± 8.8 | 64.3 ± 3.1 | 14.3 ± 4.5 | 97.5 ± 0.6 | 10.1 ± 14.3 |
| 2 | 0.5 ± 0.7 | 33.8 ± 19.9 | 8.0 ± 9.3 | 84.8 ± 5.4 | 4.1 ± 5.8 |
Purified IgG from mice primed with 104 PFU of A/New Caledonia/20/99 virus (NC99) and then boosted 4 weeks later with 103 PFU of A/California/04/09 (Cal09) virus.
Purified IgG from mice primed with 104 PFU of A/New Caledonia/20/99 virus and then boosted 4 weeks later with 105 PFU of A/Solomon Islands/3/06 (SI06) virus.
Purified IgG from mice infected with 104 PFU of A/California/04/09 virus.
Stalk-specific monoclonal antibody 6F12 used as a positive control.
Purified IgG from naïve mice.
Stalk-reactive antibodies protect from heterologous challenge in a passive transfer experiment.
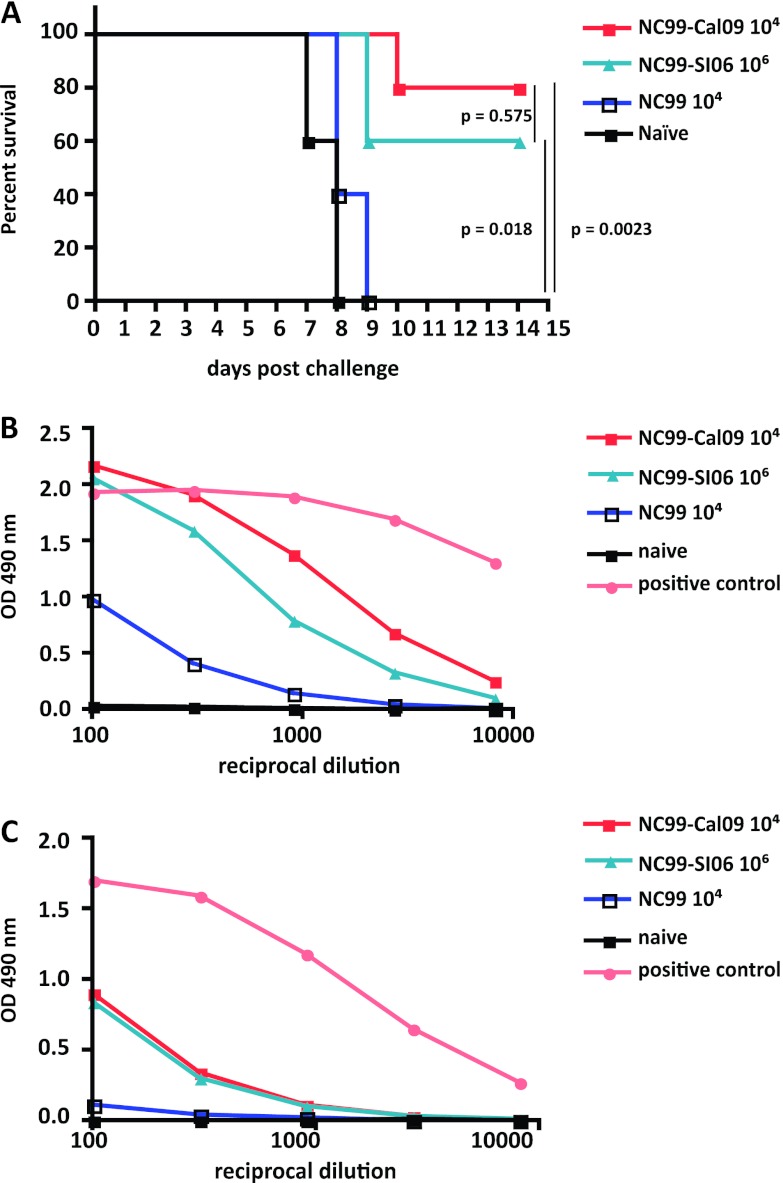
In order to assess the in vivo protective efficacy of such antibodies, sera from sequentially infected animals were administered intraperitoneally to naïve mice, followed by challenge with an H5N1 reassortant virus in the PR8 background (VN04). Animals that received sera from mice sequentially infected with seasonal and pandemic viruses (NC99, 104 PFU; Cal09, 104 PFU) were partially protected from challenge, with an 80% rate of survival. Mice that were only exposed to drifted seasonal strains (NC99, 104 PFU; SI06, 106 PFU) were also partially protected from challenge (60% survival). Mice that only received a single inoculation of NC99 virus (104 PFU) were not protected from challenge, and all succumbed to infection by day 9 (Fig. 3A).
Fig 3.
Serum from sequentially infected mice protects naïve animals from lethal H5 virus challenge and has reactivity to an H5 HA. (A) Animals were infected with 104 PFU of NC99 and then boosted 4 weeks later with 106 PFU of SI06 or 104 PFU of Cal09 virus. Serum collected from these animals was transferred intraperitoneally to naïve animals that were challenged with a lethal dose of recombinant H5 virus. Animals that received serum from animals that only experienced one NC99 infection were not protected from challenge and died with similar kinetics to controls. Serum from animals exposed to NC99 and SI06 viruses protected 60% of challenged animals, while serum from animals infected with NC99 and Cal09 viruses protected 80% of challenged mice from death. The survival rates between the NC99-SI06 and NC99-Cal09 groups were similar (P = 0.575), whereas differences in survival rates compared to the NC99-only groups were statistically significant (NC99-SI06 versus NC99, P = 0.018; NC99-Cal09 versus NC99, P = 0.0023). (B) As shown by ELISA, protection from H5 challenge correlated with serum reactivity to baculovirus-expressed H5 HA, where sera from animals that were sequentially infected had greater reactivity than sera from animals exposed to only NC99 virus. Animals that were infected with Cal09 following NC99 exposure displayed the highest degree of reactivity. Serum from animals infected with recombinant H5 expressing virus was used as a positive control. (C) Reactivity to H5N1 NA was measured by ELISA using baculovirus-expressed NA from an H5N1 virus. Animals that received two influenza virus infections had equivalent serum NA titers, while those that received only one NC99 virus infection had lower titers. Serum from animals infected with 105 PFU of a recombinant NS1-truncated H5-expressing virus was used as a positive control.
Differences in survival between the two prime-boost groups were not statistically significant (P = 0.575). However, survival of sequentially infected mice was statistically different from that of prime-only mice (NC99-SI06, P = 0.018; NC99-Cal09, P = 0.0023) and control mice (NC99-SI06, P = 0.0031; NC99-Cal09, P = 0.0031), indicating that elevated levels of stalk-reactive antibodies are able to protect from heterosubtypic challenge.
Because our findings correlated well with the degree of serum reactivity to the HA stalk for each of the experimental groups, we wanted to assess reactivity to the HA from VN04 virus. As expected, reactivity to the H5 HA was highest in the group that experienced both seasonal and pandemic virus infections (Fig. 3B). While mice that were infected with drifted seasonal strains displayed a higher degree of reactivity to the HA from VN04 virus, compared to mice inoculated only once with 104 PFU of NC99 virus, this titer was still lower than that of the NC99-Cal09-infected group, where 100-fold less virus was used for the boost (Fig. 3B). In order to rule out the potential contribution of N1-specific antibodies to the degree of protection seen here, serum antibody titers were assessed by ELISA. Titers were equivalent irrespective of the virus used to boost the initial NC99 virus infection. Boosting with a seasonal or pandemic H1N1 virus similarly enhanced the NA antibody titer over the response seen following one virus infection (Fig. 3C).
DISCUSSION
Following the emergence of the 2009 H1N1 novel swine pandemic influenza virus (pH1N1), the circulating seasonal H1N1 (sH1N1) strains were essentially replaced in the human reservoir within a year. A similar phenomenon has been seen following the introduction of other pandemic virus strains into humans, whereby the new pandemic strain replaced an influenza virus subtype that had been previously circulating (12). It has been hypothesized that the exposure to a dramatically different HA stimulates a memory response to regions that are conserved within the molecule, such as portions of the stalk, and that these antibodies reactive to the HA stalk contribute to the disappearance of the antecedent virus (12). It has been shown previously that exposure to the 2009 pH1N1 virus stimulates production of antibodies to conserved epitopes within the influenza virus HA, including those within the stalk domain of the HA (21). This suggests that antistalk antibodies, or possibly the combination of anti-globular head and antistalk antibodies, are more effective against prepandemic seasonal influenza virus strains than against the 2009 pH1N1 strain. Using cHA soluble proteins and cHA-expressing viruses, we have provided additional evidence to support this theory, demonstrating that individuals infected with the pH1N1 virus have higher stalk-specific antibody titers than uninfected controls (13). Here, we show that these findings can be recapitulated in mice, whereby infection with a seasonal H1N1 virus followed by exposure to the pandemic H1N1 strain can boost HA stalk antibody titers to a greater extent than sequential infection with drifted seasonal H1N1 viruses.
Our data support the notion that the generation of antibodies with specificity to the HA stalk domain is greatly enhanced by viral replication. Multiple factors like intracellular replication, activation of pattern recognition receptors, and antiviral signaling or the fact that more replication simply leads to more antigen presentation might be involved in the observed phenomenon. A single inoculation of sH1N1 or pH1N1 viruses generated stalk antibodies, while vaccination with inactivated preparations did not (Fig. 1A and B). While DNA vaccination is thought to mimic natural infection, in that viral proteins are translated in vivo, stalk antibodies were not detected in animals following a single DNA electroporation. The same was true when mice were infected with influenza viruses that were attenuated via cold adaptation or NS1 truncation (Fig. 1C). It is possible that stalk antibodies could be generated by sequential exposures to inactivated preparations or with higher inocula of attenuated viruses, for example, as our laboratory has generated broadly reactive mouse monoclonal antibodies directed to the hemagglutinin stalk following sequential DNA immunizations followed by a virus boost (17, 20).
Following inoculation with a second virus, the stalk antibody titer was boosted in animals infected with sH1N1 or pH1N1 viruses. The generation of stalk-specific antibodies was dose dependent, however, as animals that received a lower dose of virus (103 PFU of Cal09, 105 PFU of SI06) had a weaker boost than animals that received higher inocula (104 PFU Cal09, 106 PFU SI06) (Fig. 2). However, boosting with Cal09 virus, at doses 100- to 1,000-fold lower than those of SI06, was a more effective inducer of stalk titer. Because of the role that replication could be playing in this phenomenon, we assessed viral titers in the lungs of mice 3 days postboost. Virus was not detected in animals that had been boosted with SI06 virus. Because NC99 and SI06 HAs are 97% identical, it is possible that antibodies generated from the primary infection expeditiously cleared the virus from the mouse lung. In contrast, 105-PFU/ml titers were found in the lungs of animals boosted with either 103 or 104 PFU of Cal09 virus. The higher induction of stalk titers in these animals could therefore be a result of (i) a better ability to replicate in NC99-primed animals, (ii) the fact that its globular head domain is substantially different from the NC99 globular head domain and only memory B cells directed to conserved regions are boosted, or (iii) both factors. It is possible that the boost in antistalk titers seen in pH1N1-infected patients is governed by the same mechanism.
It is interesting to note that while animals exposed only once to influenza virus were able to produce a polyclonal response specific to the HA stalk, these total IgGs were not as efficient in neutralizing influenza virus in an in vitro assay, where the amount of IgG used was standardized between groups (Table 2). A boost therefore increases the level of stalk antibodies in vivo and might also increase the percentage of stalk-reactive antibodies in the total IgG preparation. There is also a possibility that sequential infection promotes affinity maturation, resulting in more potent antibodies. Interestingly, differences in stalk antibody titers seen between infection regimens can be more pronounced by ELISA than when assessed by a neutralization assay. This might indicate that the proportion of binding and neutralizing antibodies versus binding and nonneutralizing antibodies may depend on the infection regimen. The work presented here demonstrates that antistalk antibodies can be elicited as a result of multiple exposures to influenza virus. In particular, we can recapitulate the phenomenon seen in humans, whereby infection with pH1N1 virus stimulates the production of antibodies with specificity to the HA stalk. Because stalk-specific antibodies are known to be protective in the face of a variety of influenza virus challenges, further insight into the mechanism modulating this response would be helpful in the development of vaccines that elicit antibodies by a similar mechanism.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anice Lowen for the generation of the A/Ann Arbor/6/60 rescue system and Chen Wang for excellent technical assistance. We also thank Randy Albrecht for mouse sera that were used as a positive control.
G.S.T. was supported by National Institutes of Health (NIH) grant HHSN266200700010C and NIH training grant 1T32 AI07647. F.K. was supported by an Erwin Schrödinger Fellowship (J 3232) from the Austrian Science Fund (FWF). This work was partially supported by PATH and by CEIRS (Centers for Excellence for Influenza Research and Surveillance; HHSN26620070010C).
Footnotes
Published ahead of print 11 July 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Corti D, et al. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856 [DOI] [PubMed] [Google Scholar]
- 2. Ekiert DC, et al. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ekiert DC, et al. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans MJ, et al. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446:801–805 [DOI] [PubMed] [Google Scholar]
- 5. Fodor E, et al. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 73:9679–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hai R, et al. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol. 86:5774–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jungbauer A, et al. 1989. Comparison of protein A, protein G and copolymerized hydroxyapatite for the purification of human monoclonal antibodies. J. Chromatogr. 476:257–268 [DOI] [PubMed] [Google Scholar]
- 8. Kashyap AK, et al. 2008. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc. Natl. Acad. Sci. U. S. A. 105:5986–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kopecky-Bromberg SA, et al. 2009. Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine 27:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okuno Y, Isegawa Y, Sasao F, Ueda S. 1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67:2552–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palese P, Shaw ML. 2006. Orthomyxoviridae: the viruses and their replication, p 1648–1689 In Knipe D, Howley P. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 12. Palese P, Wang TT. 2011. Why do influenza virus subtypes die out? A hypothesis. mBio 2(5):e00150–11 doi:10.1128/mBio.00150-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pica N, et al. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. U. S. A. 109:2573–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steel J, et al. 2009. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J. Virol. 83:1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steel J, et al. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1(1):e00018–10 doi:10.1128/mBio.00018-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sui J, et al. 2009. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan GS, et al. 2012. A pan-h1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J. Virol. 86:6179–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Throsby M, et al. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942 doi:10.1371/journal.pone.0003942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S, et al. 2008. Simplified recombinational approach for influenza A virus reverse genetics. J. Virol. Methods 151:74–78 [DOI] [PubMed] [Google Scholar]
- 20. Wang TT, et al. 2010. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 6:e1000796 doi:10.1371/journal.ppat.1000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wrammert J, et al. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.