Abstract
Pantoea stewartii subsp. stewartii, the causal agent of Stewart's wilt of sweet corn, produces a yellow carotenoid pigment. A nonpigmented mutant was selected from a bank of mutants generated by random transposon mutagenesis. The transposon insertion site was mapped to the crtB gene, encoding a putative phytoene synthase, an enzyme involved in the early steps of carotenoid biosynthesis. We demonstrate here that the carotenoid pigment imparts protection against UV radiation and also contributes to the complete antioxidant pathway of P. stewartii. Moreover, production of this pigment is regulated by the EsaI/EsaR quorum-sensing system and significantly contributes to the virulence of the pathogen in planta.
INTRODUCTION
Pantoea stewartii subsp. stewartii (formerly Erwinia stewartii) is a yellow-pigmented, Gram-negative bacterial phytopathogen that causes a severe disease of sweet corn (Zea mays) called Stewart's wilt. The bacterium is introduced into the plant by its insect vector, the corn flea beetle (Chaetocnema pulicaria), where it colonizes both the apoplast and the xylem. Following systemic colonization of the plant, the bacteria exit the leaf tissue as a visible, yellow bacterial ooze. It is the preferential colonization of the xylem that blocks water flow in the plant and leads to the characteristic wilting associated with the disease. The type III secretion system, stewartan exopolysaccharide production, flagellum-based motility, and one host cell wall-degrading enzyme have been implicated as important pathogenicity or virulence factors for P. stewartii, but little is known about the biological role of the characteristic yellow pigment produced by P. stewartii (4, 10, 11, 22, 35).
Carotenoids are among the most diverse natural products; they are synthesized by many organisms, including animals, plants, and microorganisms, and absorb light in the 400- to 550-nm range, which gives them their yellow-orange color (5). Several Erwinia species, which are close relatives of P. stewartii, produce yellow carotenoid pigments and possess a conserved carotenoid biosynthesis operon consisting of the genes crtE, crtX, crtY, crtI, and crtB in map order. Phytoene synthase, encoded by crtB, is the enzyme for the first step in carotenoid biogenesis, and mutations in crtB render a nonpigmented phenotype (36, 40, 52). More specifically, the P. stewartii genome contains a conserved carotenoid biosynthesis operon found in Erwinia spp., where crtE encodes geranylgeranyl pyrophosphate synthase, crtX encodes 3-hydroxy-β-carotene glycosylase, crtY encodes lycopene cyclase, and crtI encodes phytoene dehydrogenase (43, 46). The crtB gene encodes phytoene synthase, which converts geranylgeranyl pyrophosphate to phytoene, an early step in the biosynthesis of β-carotene (41). Zeaxanthin diglucoside, a derivative of β-carotene, is the typical carotenoid produced by Erwinia spp. (2), and because of the high homology to the carotenoid biosynthetic operon in other Erwinia spp., we speculate that the P. stewartii likely produces zeaxanthin diglucoside or a closely related derivative.
Carotenoids are known antioxidants that can protect against various types of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), hydroxyl radicals, and superoxide anions (7, 24, 29). Carotenoids can also quench singlet oxygen (1O2) (9, 24, 45). ROS can also be derived from the absorption of visible light by chlorophyll present in the plant host (47). Several plant-associated bacteria, including Erwinia herbicola, synthesize carotenoids, which aid in the defense against the deleterious effects of ROS generated by chlorophyll during photosynthesis (38a). Carotenoids can also act as photoprotectants against the damage incited by UV radiation, specifically, by providing protection against near-UV (320- to 400-nm) wavelengths (46–48). Not surprisingly, carotenoids also play a role in overall fitness (26) and virulence within the host for some species of pathogenic bacteria, such as Cronobacter sakazakii and Staphylococcus aureus (33).
The objective of this study was to investigate the biological role of carotenoid pigment production in P. stewartii subsp. stewartii, specifically regarding tolerance to oxidative stress, UV radiation, and virulence in the sweet corn host.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
P. stewartii and Escherichia coli strains were maintained on nutrient agar (Difco) and in Miller's Luria-Bertani (LB) medium (Difco) at 28°C. When necessary, antibiotics were added to the media at the following concentrations: nalidixic acid, 30 μg/ml; spectinomycin, 100 μg/ml; and ampicillin, 100 μg/ml. Descriptions of the bacterial strains and plasmids used in this study are presented in Table 1.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotypea | Reference or source |
|---|---|---|
| Strains | ||
| P. stewartii subsp. stewartii | ||
| DC283 | SS104 WT, Nalr | 12 |
| MM13 | crtB∷Mar2xT7 Nalr Gmr | This study |
| MM16 | MM13 harboring pBBR1∷crtB, Nalr Gmr Apr | This study |
| ESN10 | DC283 ΔesaI Nalr | 4 |
| E. coli | ||
| DH10ß | F− endA1 recA1 galE15 galK16 nupG rpsL ΔlacX74 ϕ80lacZΔM15 araD139 Δ(ara leu)7697 mcrA Δ(mrr-hsdR MS-mcrBC) λ− | Invitrogen |
| S17-1λpir+ | thi pro hsdR hsdM1 recA RP4 2-Tc∷Mu-Km∷Tn7 Smr λpir | 43a |
| Plasmids | ||
| pMAR2xT7 | mariner transposon Himar1, Gmr | 32 |
| pCR8/GW/TOPO | TA cloning entry vector, Spr | Invitrogen |
| pBBR1-MCS4-GW | pBBR1-MCS4 modified as a Gateway destination vector, Apr | 6a |
| pMOJ13 | pCR8/GW/TOPO∷crtB Spr | This study |
| pMOJ14 | pBBR1∷crtB Apr | This study |
Antibiotic resistance abbreviations: Apr, ampicillin resistance; Gmr, gentamicin resistance; Nalr, nalidixic acid resistance; Spr, spectinomycin resistance.
Identification of a transposon mutant deficient in pigment production.
Wild-type (WT) strain DC283 was mutated by random transposon insertion mutagenesis utilizing the MAR2xT7 transposon, a derivative of the mariner transposon Himar1 (32). In brief, the pMAR2xT7 suicide plasmid was introduced into WT DC283 by conjugal transfer. Transconjugants were plated onto LB agar medium containing nalidixic acid and gentamicin. Nonpigmented colonies were selected for further characterization. Arbitrary PCR was used to determine the location of the MAR2xT7 transposon insertion in the nonpigmented mutants. This is a two-round PCR protocol that amplifies the sequence adjacent to the transposon insertion using a transposon-specific primer and an arbitrary primer. A second round of PCR uses a nested transposon-specific primer and a primer that anneals to the nonrandom portion of the arbitrary primer (32). Specifically, genomic DNA from the nonpigmented mutants was extracted using the DNeasy blood and tissue kit from Qiagen (MD). The first round of PCR was performed with the TnMar1/ARB1 primer pair (Table 2) using optimized PCR conditions (32). The PCR product from the first round was used as a template for the second round of PCR with the TnMar2/ARB2 primer pair (Table 2) (32). The final PCR product was sequenced using the SeqTnMar primer (Table 2) (32).
Table 2.
Primer sequences used in this study
| Primer | Sequencea | Reference or source |
|---|---|---|
| TnMar1 | TACAGTTTACGAACCGAACAGGC | 32 |
| ARB1 | GGCCAGGCCTGCAGATGATGNNNNNNNNNNGTAT | 32 |
| TnMar2 | TGTCAACTGGTTCGTGCCTTCATCCG | 32 |
| ARB2 | GGCCAGGCCTGCAGATGATG | 32 |
| SeqTnMar | GACCGAGATAGGGTTGAGTG | 32 |
| PSF | TACAAAAAAGCAGGCTAGCTCAATGCCTGGCAAGGTT | This study |
| PSR | TACAAGAAAGCTGGGTCGCGCCACCGTTATCTAAACTTCA | This study |
Primer sequences are oriented from 5′ to 3′. Gateway sequences are shown in bold.
Complementation of the nonpigmented crtB mutant.
The crtB gene, encoding a putative phytoene synthase, was amplified using primer pair PSF/PSR (Table 2). The amplicon included 150 bp upstream of the open reading frame to ensure inclusion of the native promoter. The resulting PCR product was cloned into the pCR8/GW/TOPO TA cloning vector (Invitrogen) following the manufacturer's instructions to create plasmid pMOJ13. The crtB gene was subcloned from pMOJ13 into a Gateway-compatible version of the broad-host-range vector pBBR1-MCS4 (28) using the LR Clonase kit II (Invitrogen) according to the manufacturer's instructions to create pMOJ14. pMOJ14 was electroporated into the MM13 (crtB∷Mar2xT7) mutant, and transformants were selected on nutrient agar plates containing nalidixic acid and ampicillin.
Pigment extraction and spectral analysis.
Single colonies of WT DC283 or MM13 (crtB∷Mar2xT7) were grown in 2 ml LB medium with shaking (200 rpm) at 28°C for 48 h. Bacteria were harvested by centrifugation at 15,000 rpm. The resulting bacterial pellet was washed once in sterile distilled H2O and mixed with 1 ml of 100% methanol, based on a previously described protocol (42). The suspension was heated at 85°C for 20 min to extract the pigment. This mixture was centrifuged, the supernatant was transferred to a fresh tube, and these methanol extracts were scanned at between 400 and 500 nm using a Biomate 3 spectrophotometer (Thermo Fisher).
Sensitivity to UV radiation.
Sensitivity to UV radiation was tested based on a previously described protocol (39). In brief, bacterial strains were cultured in 2 ml LB broth to either mid-log phase or stationary phase, serially diluted in 1× phosphate-buffered saline (PBS), and placed in 13- by 100-mm glass tubes. The tubes were placed in a rack over a UV transilluminator and exposed to near-UV (320- to 400-nm) in an upright position for 30 s. Unexposed serial dilutions were used as a negative control. Aliquots (100 μl) of the exposed or unexposed cells were spread plated on nutrient agar containing the appropriate antibiotics. Colonies were counted after 3 days of incubation at 28°C. Results were log transformed and expressed as percent surviving CFU normalized to that for the untreated control.
H2O2 and singlet oxygen sensitivity assays.
For the H2O2 sensitivity assays, single colonies of WT DC283, MM13 (crtB∷Mar2xT7), and MM16 (crtB∷Mar2xT7/pMOJ14) were grown in 2 ml LB broth to either mid-log phase or stationary phase. Two hundred microliters of bacterial cell culture was diluted with 200 μl LB broth in a sterile glass tube, and H2O2 was added to a final concentration of 40 mM as previously described (38). Controls were cell suspensions that were not treated with H2O2. The tubes were shaken at 200 rpm at 28°C for 30 min, stored on ice, and immediately serially diluted in 1× PBS. Survivability was assessed by spread plating 100-μl aliquots of each dilution for the H2O2-treated or untreated cells onto nutrient agar plates containing antibiotics where needed. Colonies were enumerated after 3 days of incubation at 28°C. The results were expressed as log-transformed CFU/ml normalized to that for the untreated control.
Toluidine blue generates singlet oxygen (1O2) upon illumination with tungsten light (25). To test sensitivity to 1O2, bacterial strains were cultured in LB broth to mid-log phase or stationary phase and then serially diluted in 1× PBS buffer. Serial dilutions were treated with toluidine blue to a final concentration of 5 μM in 13- by 100-mm glass culture tubes. The tubes were placed on a rack, positioned 60 cm from two 150-W tungsten light bulbs at 28°C, and left for 1 h with occasional shaking. Aliquots of the cell suspensions were spread plated on nutrient agar containing 30 μg/ml nalidixic acid. Separate tubes containing the various serial dilutions were left untreated and served as negative controls. Colonies were counted 3 days after incubation at 28°C. The results were expressed as log-transformed CFU/ml normalized to that for the untreated control.
3-Oxo-C6-AHL induction of pigment production.
Single colonies of the N-3-(oxohexanoyl)-l-homoserine lactone (3-oxo-C6-AHL) signal synthase mutant ESN10 (ΔesaI) (4) were grown in LB medium to an optical density at 600 nm (OD600) of 0.1 at 28°C with shaking at 200 rpm. One set of cultures were supplemented with 3-oxo-C6-AHL (Sigma-Aldrich, St. Louis, MO) to a final concentration of 10 μM. The other set of cultures did not receive exogenous 3-oxo-C6-AHL. Following this, all cultures were grown to stationary phase, and the pigment was extracted and quantified as described above.
Virulence assays.
Fourteen-day-old sweet corn seedlings (var. Jubilee) (Syngenta) were inoculated by stabbing the stem below the secondary leaf using a sterile 20-gauge syringe needle and placing 5 μl of bacterial inoculum into the wound. Inocula were prepared by growing single colonies of WT DC283, MM13 (crtB∷Mar2xT7), and MM16 (crtB∷Mar2xT7/pMOJ14) overnight. The cells were harvested by centrifugation, washed once with 1× PBS containing 0.2% Tween 20 (1× PBS-Tween 20), and resuspended in the same buffer to an OD600 of 1.0. Seedlings were assessed daily for symptom development using the following arbitrary rating scale: 0, no symptoms; 1, small water-soaked lesions; 2, larger water-soaked lesions; 3, water-soaked lesions, ooze formation, and slight wilting; 4, severe wilting and ooze; and 5, dead. Seedlings inoculated with 1× PBS-Tween 20 buffer served as negative controls.
RESULTS
Identification of a mutation affecting pigment production.
Following random transposon mutagenesis, several nonpigmented mutants were selected. We focused on one mutant in particular (Fig. 1), where the transposon mapped to the crtB gene (insertion at 680 bp), which encodes a putative phytoene synthase. The closest orthologs to the P. stewartii crtB gene are crtB in Pantoea ananatis (accession no. YP 003522457.1) (E = 0.0; 88% identity with 99% coverage) (15) and crtB in Pantoea agglomerans (accession no. AAA21264.1) (E = 0.0; 88% identity with 95% coverage) (46). A closer look at the genomic context of the crtB gene in the P. stewartii genome revealed that it was the last gene in a putative operon containing crtEXYIB (accession no. AY166713.1).
Fig 1.
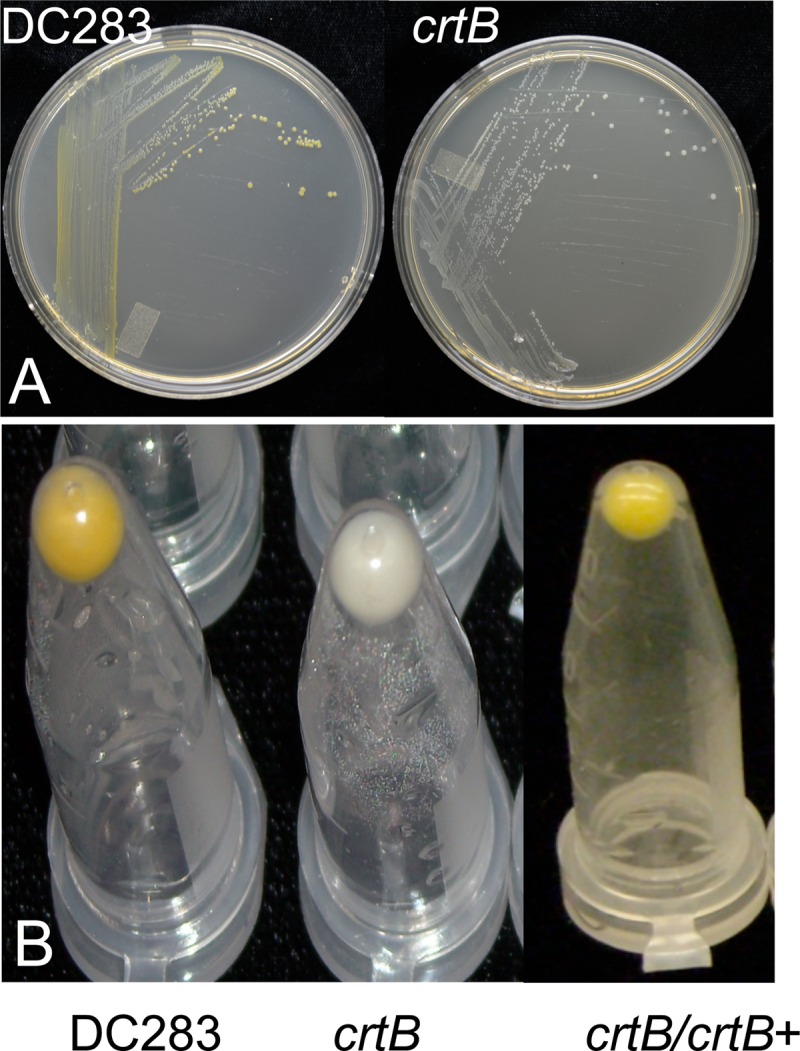
Pigment production by wild-type P. stewartii (DC283) and the crtB∷Mar2xT7 (crtB) mutant. (A) Single colonies of the crtB transposon mutant obtained were nonpigmented on nutrient agar. (B) Bacterial pellets accumulated a yellow carotenoid pigment in the wild-type strain DC283 but not the crtB mutant. Pigmentation was restored when a wild-type copy of crtB was supplied in trans on plasmid pMOJ14 in strain crtB∷Mar2xT7/pMOJ14 (crtB/crtB+).
Carotenoid analysis.
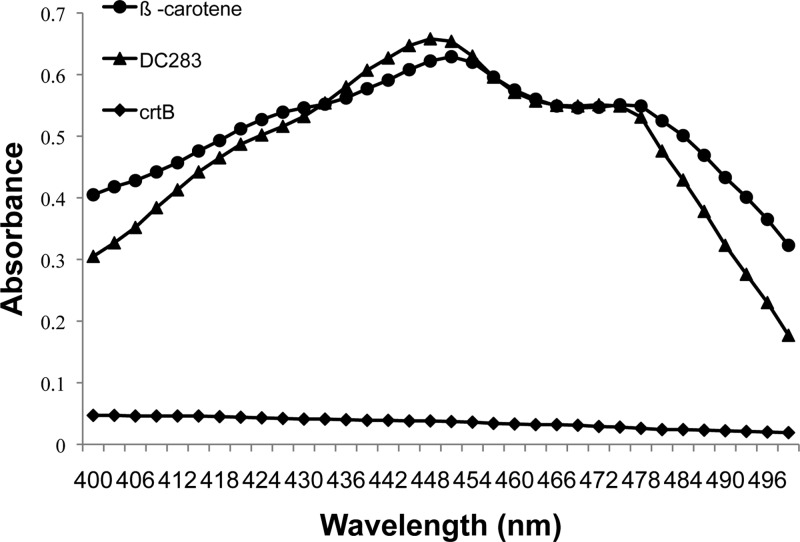
The absorption spectrum of the methanol-extracted pigment of WT P. stewartii (DC283) displayed a typical carotenoid spectrum in the visible range, with a peak at 451 nm, a shoulder at 475 nm, and the maximum absorbance (λmax) at 450 nm, which was comparable to that of the β-carotene standard (Fig. 2) (51). As expected, the crtB mutant had minimal detectable carotenoid in the methanol extract (Fig. 2).
Fig 2.
Spectral analysis of the P. stewartii carotenoid pigment. The methanol-extractable pigment from wild-type P. stewartii (DC283) has an absorption spectrum with a peak at 451 nm and a shoulder at 475 nm and with the maximum absorbance (λmax) at 450 nm, which was similar to that of the β-carotene standard. The crtB∷Mar2xT7 (crtB) mutant did not have detectable pigment in the methanol extract.
The P. stewartii pigment functions as an antioxidant.
At mid-log phase, the MM13 (crtB∷Mar2xT7) strain was significantly more sensitive to H2O2 treatment than WT DC283 (Fig. 3). Survivability was quantified at 20% for the mutant and 34% for WT DC283. H2O2 tolerance was restored to wild-type levels in the MM13 (crtB∷Mar2xT7) mutant when a wild-type copy of crtB was supplied on plasmid pMOJ14. The results represent the means ± standard errors (SE) from at least three independent experiments, each containing three technical replicates, and a Student t test was used to determine the significance of differences among the different treatments. Interestingly, there was no difference in sensitivity to H2O2 treatment when both the MM13 (crtB∷Mar2xT7) mutant and WT DC283 were grown to stationary phase (data not shown). The loss of pigment production did not lead to a significant change in susceptibility to 1O2 generated by toluidine blue treatment in cells grown to mid-log or stationary phase, indicating that the carotenoid pigment does not play a significant role in quenching 1O2 in P. stewartii (data not shown).
Fig 3.
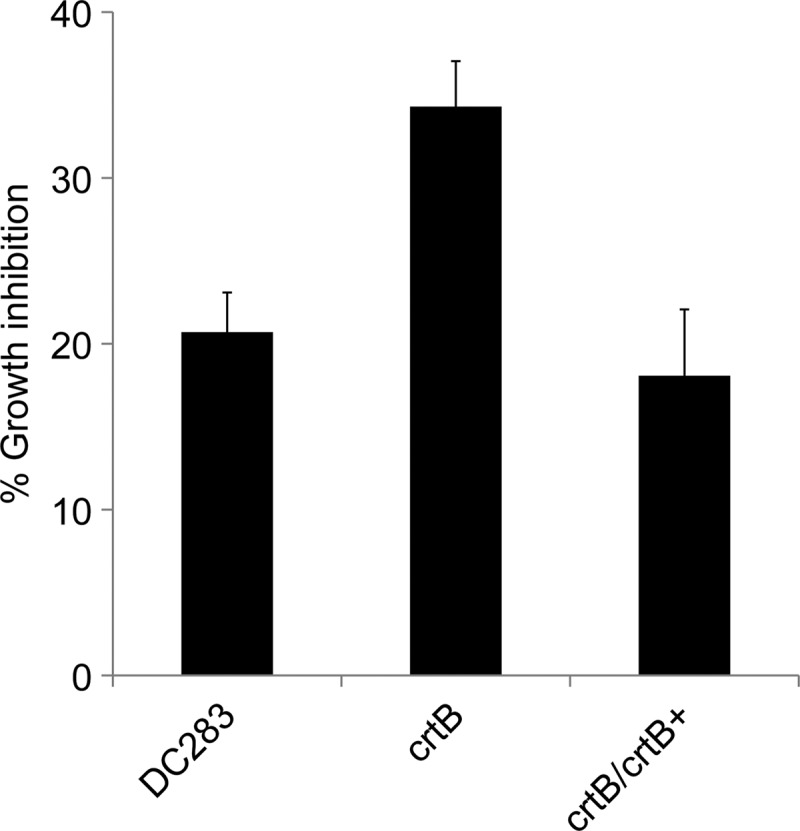
H2O2 sensitivity in the wild-type P. stewartii (DC283), the crtB∷Mar2xT7 (crtB) mutant, and the crtB∷Mar2xT7/pMOJ14 (crtB/crtB+) complemented strain. Mid-log-phase cells were treated with H2O2 to a final concentration of 40 mM for 30 min. Controls were left untreated with H2O2. CFU were enumerated, and the percent growth inhibition was normalized to that for the untreated control. The crtB∷Mar2xT7 (crtB) mutant was significantly more sensitive to H2O2 treatment than WT DC283. H2O2 tolerance was fully restored when a wild-type copy of crtB was supplied in trans on plasmid pMOJ14 in strain crtB∷Mar2xT7/pMOJ14 (crtB/crtB+).
The P. stewartii pigment protects against UV radiation.
WT DC283 and MM13 (crtB∷Mar2xT7) were equally susceptible to short-wave UV treatment during mid-log phase (data not shown). However, when the culture entered stationary phase, WT DC283 was significantly more tolerant to UV stress than the MM13 (crtB∷Mar2xT7) mutant, indicating that the carotenoid pigment plays an important role in protecting the cells from the harmful effects of UV radiation during stationary phase rather than log phase (Fig. 4). The results represent the means ± SE from at least three independent experiments, each containing three technical replicates, and a Student t test was used to determine the significance of differences among the different treatments.
Fig 4.
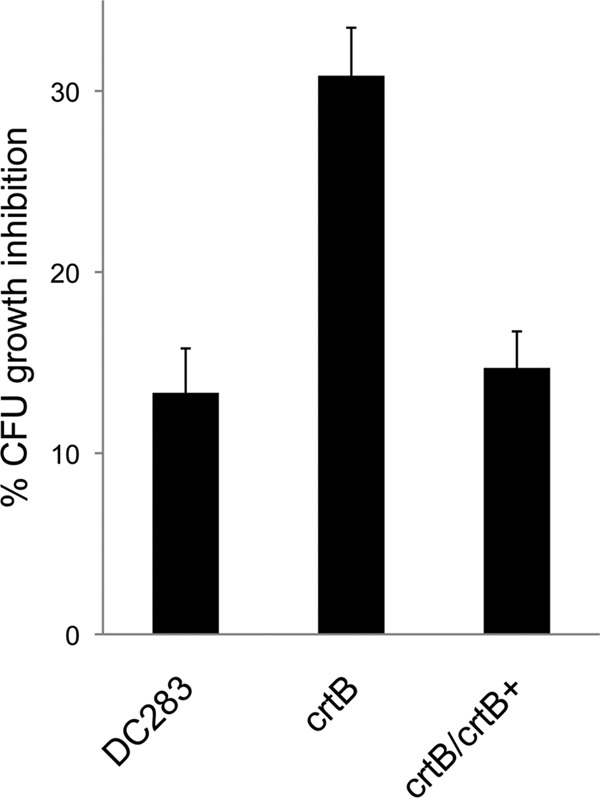
Tolerance to UV radiation. Wild-type P. stewartii (DC283), the crtB∷Mar2xT7 (crtB) mutant, and the crtB∷Mar2xT7/pMOJ14 (crtB/crtB+) complemented strain were grown to stationary phase in LB medium, diluted in 1× PBS, and exposed to near-UV-A irradiation (320 to 400 nm) for 30 s. CFU were enumerated, and the percent growth inhibition was normalized to that for the untreated control. The crtB∷Mar2xT7 (crtB) mutant was significantly more sensitive to UV radiation than the WT (DC283) strain, and UV tolerance was fully restored in the crtB∷Mar2xT7/pMOJ14 (crtB/crtB+) complemented strain.
P. stewartii produces pigment in a quorum-sensing-dependent manner.
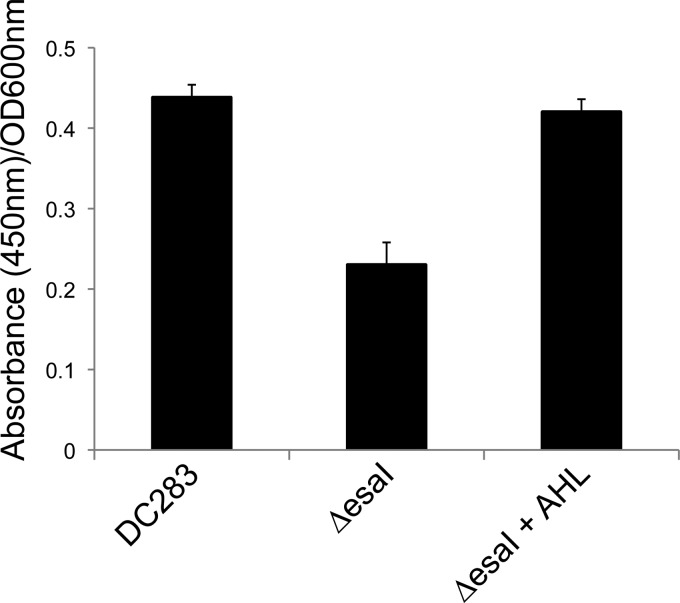
We observed that cultures of P. stewartii produced pigment in mid-log phase but accumulated the bulk of the pigment in late log/early stationary phase (data not shown), suggesting to us that pigment production occurs in a cell density-dependent manner. This prompted us to test if pigment production was dependent on the EsaI/EsaR quorum-sensing system. The quorum-sensing system depends on the production of a 3-oxo-C6-AHL signal that is perceived by the cell density-responsive transcriptional regulator protein, EsaR. EsaI is the signal synthase, and a ΔesaI mutant is deficient in signal production (49). The ΔesaI signal synthase mutant produced significantly less pigment than WT DC283, and pigment production was fully restored when exogenous 3-oxo-C6-AHL signal was added to the medium, demonstrating that the EsaI/EsaR system is involved in the regulation of pigment production in P. stewartii either directly or indirectly (Fig. 5). The results represent the means of nine technical replicates from three independent experiments ± SE. A Student t test was used to determine the significance of differences among the different treatments.
Fig 5.
The EsaI/EsaR quorum-sensing system regulates carotenoid pigment production. Wild-type P. stewartii (DC283) and the ΔesaI quorum-sensing signal synthase mutant, with or without exogenous 3-oxo-C6-AHL (AHL) signal, were grown in LB medium for 48 h. Pigment extraction and quantification were performed as described in Materials and Methods. The ΔesaI mutant produces less pigment, and pigment production is restored when the AHL signal is added to the medium.
Loss of pigment production leads to a reduction in virulence.
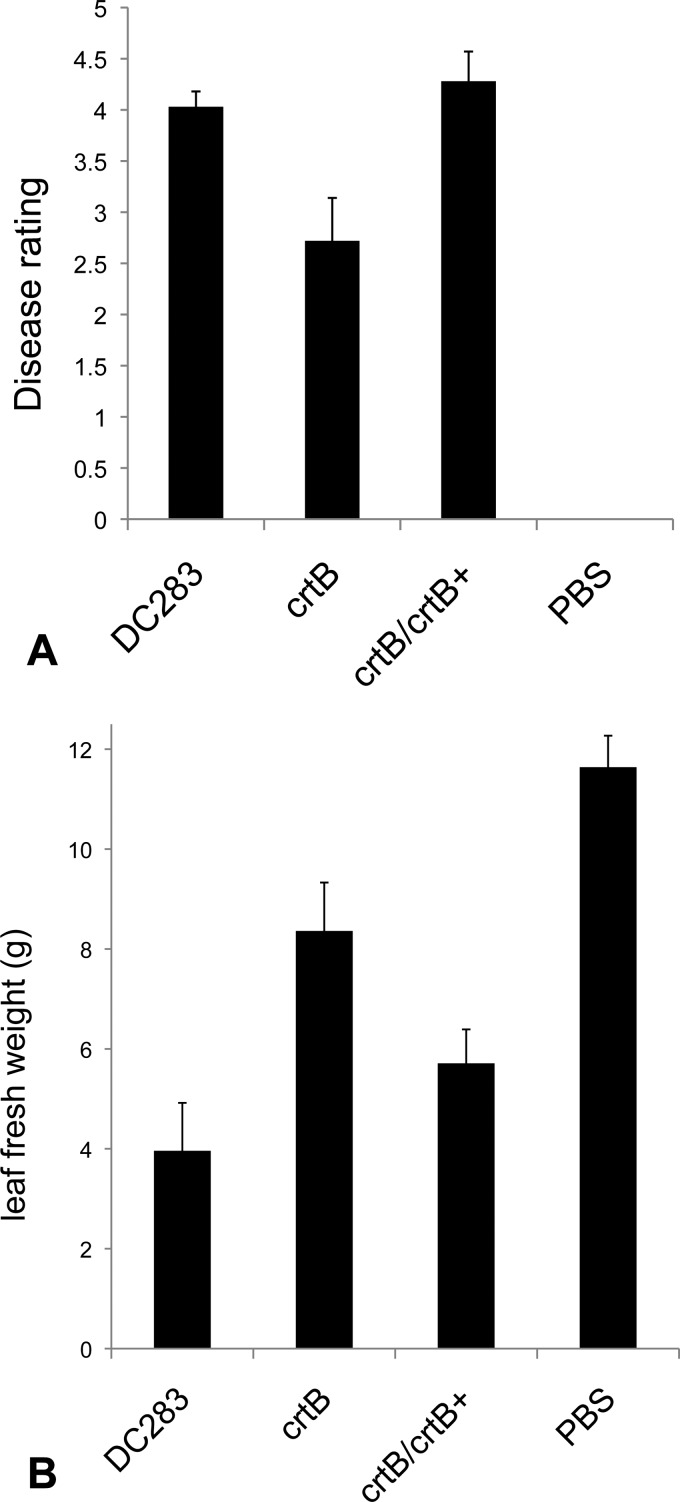
The WT DC283 parental strain was fully virulent and caused the formation of both water-soaked lesions and wilting, characteristic of the disease. At 10 days after inoculation, these plants rated a 4.2 on the arbitrary disease rating scale of 0 to 5 (Fig. 6). In contrast, plants inoculated with the MM13 (crtB∷Mar2xT7) mutant were significantly delayed in both lesion development and wilting. The symptoms of these plants gave an average disease rating of 2.7 (Fig. 6). Virulence was fully restored when the mutant strain was complemented with a functional copy of crtB, carried on plasmid pMOJ14 in strain MM16 (crtB∷Mar2xT7/pBBR1∷crtB) (Fig. 6). For each experiment, the results represent the mean of 18 technical replicates from three independent experiments ± SE. A Student t test was used to determine the significance of differences among the different treatments. These data demonstrate that carotenoid pigment production is required for full virulence for P. stewartii.
Fig 6.
(A) Virulence assay in sweet corn seedlings. Fourteen-day-old sweet corn seedlings were inoculated with either the wild-type P. stewartii (DC283), the crtB∷Mar2xT7 (crtB) mutant, the crtB∷Mar2xT7/pMOJ14 (crtB/crtB+) complemented strain, or 1× PBS-Tween 20 buffer. Seedlings were stab inoculated below the secondary leaf with 5 μl of inoculum prepared in 1× PBS-Tween 20 buffer and adjusted to an OD600 of 1.0. Disease ratings were assessed at 10 days postinoculation. The crtB∷Mar2xT7 (crtB) mutant is significantly comprised in virulence compared to the WT (DC283) strain. Virulence was fully restored in the crtB∷Mar2xT7/pMOJ14 (crtB/crtB+) complemented strain. Plants inoculated with 1× PBS-Tween 20 buffer alone did not develop any Stewart's wilt symptoms. (B) Total fresh weight (g) of sweet corn seedlings. Plants were weighed 12 days post-inoculation. The results represent the means ± SE for 12 replicates from three independent experiments.
DISCUSSION
ROS, such as H2O2 and 1O2 oxygen, are damaging to cellular membranes, protein, and DNA. Bacteria have evolved an effective oxidative stress response that includes the production of ROS-detoxifying enzymes such as catalases, alkyl hydroperoxidases, and superoxide dismutases (44). Another line of defense against oxidative stress includes the production of antioxidant carotenoid pigments that can effectively quench ROS (29, 30). Plants produce ROS during the host defense response as part of the oxidative burst, which is a rapid, transient response to pathogen invasion (1, 6, 21, 27, 31, 51). The oxidative burst is derived from living plant cells; thus, P. stewartii would likely encounter this stress in the early apoplastic (water-soaked lesion formation) phase of infection. In addition to the apoplastic phase, P. stewartii preferentially colonizes the xylem tissue as it systemically colonizes the plant, and bacterial pathogens can also incur oxidative stress in the xylem (17). Xylem tissue cells are nonliving and therefore do not initiate an oxidative burst on their own. However, pathogens in the xylem do make contact with adjacent living parenchyma cells, and ultrastructural studies indicate that these cells can sense xylem-invading pathogens and mount a defense response that includes the production of ROS (23). Another source of ROS comes from differentiating thin-walled xylem cells and particular nonlignifying xylem parenchyma cells. These cells are capable of sustained H2O2 production, which is important for the cross-linking that occurs during the lignification process of developing xylem elements that can diffuse widely to neighboring xylem cells (3, 18, 19).
Once P. stewartii enters the xylem, it proliferates and reaches high cell densities (≥108 CFU/g tissue). Our results demonstrate that production of the carotenoid pigment by P. stewartii confers tolerance to H2O2. Interestingly, the protective effects against H2O2 were observed only in mid-log-phase cells and not in cells grown to stationary phase, indicating the importance of the role of the carotenoid pigment as an antioxidant in a rapidly growing population such as occurs when the bacteria are colonizing the xylem tissue. This also suggests that other antioxidant mechanisms, perhaps controlled by the stationary-phase stress response sigma factor RpoS, are dominant to the antioxidant capabilities of the carotenoid pigment during stationary phase (8). We also demonstrated that pigmentation does not play an apparent role in quenching 1O2 for P. stewartii. 1O2 can arise as a by-product of photosynthesis. P. stewartii preferentially resides in the xylem tissue, which is devoid of chloroplasts; therefore, the ability to detoxify 1O2 may not be necessary for P. stewartii in the specialized niche of the xylem.
Our data show that pigment production in P. stewartii is under the control of the EsaI/EsaR quorum-sensing system and is most abundant in a high-cell-density situation. When the bacteria are initially introduced into the plant by the corn flea beetle, they are at low cell density and would not be producing maximal amounts of pigment. Therefore, we reason that the pigment is not a large contributor to combating oxidative stress associated with the oxidative burst when small amounts of bacteria (low cell density) are initially introduced into the plant by the insect vector. Rather, we speculate that the protective antioxidant effects of the pigment are more important during the xylem colonization phase of the disease, where the bacterial population reaches the cell numbers necessary for the EsaI/EsaR quorum-sensing system to initiate gene expression of known quorum-sensing-regulated genes (50) and, specifically, pigment production. In addition to acting as potent antioxidants, carotenoids can also provide significant protection against near-UV (320 to 400 nm) and photosensitizing agents (activated by UV), which helps to prevent photodamage (16, 47). Carotenoids are hydrophobic and generally localized to hydrophobic regions of the cell, such as membranes (5). We postulate that the P. stewartii pigment accumulates in the membrane, where it would be optimally located to serve as a photoprotectant for the bacterial cell. Following systemic colonization of the plant to high cell density during the late stages of the P. stewartii infection process, substantial amounts of bright yellow bacterial ooze exits the leaf tissue and accumulates on the leaf surface. We have demonstrated that the carotenoid pigment produced by P. stewartii imparts tolerance to near-UV radiation in the stationary phase of growth, and we reasoned that P. stewartii utilizes pigment protection as a defense against the harmful effects of UV radiation as it enters stationary phase late in the disease cycle, exits the leaf tissue, and waits to be acquired by the corn flea beetle vector (13).
Pigments, in general, can play a role in virulence in bacterial pathogens (20, 34, 37). Specifically, a carotenoid pigment called staphyloxanthin is a known virulence factor for S. aureus and is even referred to as its “golden coat” (14). This pigment promotes virulence via its antioxidant activity by contributing to survivability against the oxidative burst derived from human neutrophils and whole mouse blood (33). In addition, pigment-deficient mutants were less virulent in a mouse model (33, 34). Likewise, we found that absence of the carotenoid decreases virulence for P. stewartii, demonstrating the importance of this pigment in the pathogenic lifestyle of this bacterium.
This study indicates the importance of carotenoid pigment production in virulence for P. stewartii, a xylem-dwelling phytopathogen, and points at different aspects of the disease cycle where its antioxidant and UV properties may be important. We envision a model where the carotenoid pigment contributes to the antioxidant defense system of P. stewartii as it is colonizing the xylem and reaches the cell densities necessary for the EsaI/EsaR quorum-sensing system to trigger gene expression. Once the bacteria have heavily colonized the plant and presumably reached stationary phase, they emerge from the leaf tissue producing maximal amounts of pigment, which is visible as a bright yellow bacterial ooze and aids the cells in coping with UV light exposure encountered on the leaf surface. Future studies will test this model and may possibly identify a role of this pigment in epiphytic survival of this important pathogen of sweet corn.
ACKNOWLEDGMENTS
This work was supported by an award to M. Caroline Roper from the Regents of the University of California, Riverside, and the Agricultural Experiment Station and College of Natural and Agricultural Sciences.
We thank Susanne von Bodman (National Science Foundation) for kindly providing us with the ESN10 (ΔesaI) mutant used in this study.
Footnotes
Published ahead of print 20 July 2012
REFERENCES
- 1. Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55:373–399 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong GA, Hearst JE. 1996. Carotenoids. 2. Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 10:228–237 [DOI] [PubMed] [Google Scholar]
- 3. Barcelo AR. 2005. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 220:747–756 [DOI] [PubMed] [Google Scholar]
- 4. Beck von Bodman S, Farrand SK. 1995. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J. Bacteriol. 177:5000–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Britton G. 1995. Structure and properties of carotenoids in relation to function. FASEB J. 9:1551–1558 [PubMed] [Google Scholar]
- 6. Buchanan BB, Gruissem W, Jones RL. 2000. Biochemistry and molecular biology of plants, p 52–100 American Society of Plant Physiologists, Rockville, MD [Google Scholar]
- 6a. Carlier A, Burbank L, von Bodman SB. 2009. Identification and characterization of three novel EsaI/EsaR quorum-sensing controlled stewartan exopolysaccharide biosynthetic genes in Pantoea stewartii ssp. stewartii. Mol. Microbiol. 74:903–913 [DOI] [PubMed] [Google Scholar]
- 7. Chen CH, et al. 2011. Direct observation of the beta-carotene reaction with hydroxyl radical. J. Phys. Chem. 115:2082–2089 [DOI] [PubMed] [Google Scholar]
- 8. Chiang SM, Schellhorn HE. 2012. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 10.1016/j.abb.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 9. Conn PF, Schalch W, Truscott TG. 1991. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. 11:41–47 [DOI] [PubMed] [Google Scholar]
- 10. Coplin DL, Frederick RD, Majerczak DR. 1992. New pathogenicity loci in Erwinia stewartii identified by random Tn5 mutagenesis and molecular cloning. Mol. Plant Microbe Interact. 5:266–268 [Google Scholar]
- 11. Coplin DL, Frederick RD, Majerczak DR, Tuttle LD. 1992. Characterization of a gene cluster that specifies pathogenicity in Erwinia stewartii. Mol. Plant Microbe Interact. 5:81–88 [Google Scholar]
- 12. Coplin DL, Frederick RD, Majerczak DR, Haas ES. 1986. Molecular cloning of virulence genes from Erwinia stewartii. J. Bacteriol. 168:619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Correa VR, et al. 2008. Characterization of a Pantoea stewartii TTSS gene required for persistence in its flea beetle vector. Phytopathology 98:S41 [Google Scholar]
- 14. Daum RS. 2008. Removing the golden coat of Staphylococcus aureus. N. Engl. J. Med. 359:85–87 [DOI] [PubMed] [Google Scholar]
- 15. De Maayer P, et al. 2010. Genome sequence of Pantoea ananatis LMG20103, the causative agent of Eucalyptus blight and dieback. J. Bacteriol. 192:2936–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ehling-Schulz M, Bilger W, Scherer S. 1997. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 179:1940–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flores-Cruz Z, Allen C. 2009. Ralstonia solanacearum encounters an oxidative environment during tomato infection. Mol. Plant Microbe Interact. 22:773–782 [DOI] [PubMed] [Google Scholar]
- 18. Gabaldon C, Lopez-Serrano M, Pedreno MA, Barcelo AR. 2005. Cloning and molecular characterization of the basic peroxidase isoenzyme from Zinnia elegans, an enzyme involved in lignin biosynthesis. Plant Physiol. 139:1138–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gabaldon C, Ros LVG, Pedreno MA, Barcelo AR. 2005. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol. 165:121–130 [DOI] [PubMed] [Google Scholar]
- 20. Goel AK, Rajagopal L, Sonti RV. 2001. Pigment and virulence deficiencies associated with mutations in the aroE gene of Xanthomonas oryzae pv. oryzae. Appl. Environ. Microbiol. 67:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harada H, Ishikawa H. 1997. Phylogenetical relationship based on groE genes among phenotypically related Enterobacter, Pantoea, Klebsiella, Serratia and Erwinia species. J. Gen. Appl. Microbiol. 43:355–361 [DOI] [PubMed] [Google Scholar]
- 22. Herrera CM, Koutsoudis MD, Wang X, von Bodman SB. 2008. Pantoea stewartii subsp. stewartii exhibits surface motility, which is a critical aspect of Stewart's wilt disease development on maize. Mol. Plant Microbe Interact. 21:1359–1370 [DOI] [PubMed] [Google Scholar]
- 23. Hilaire E, et al. 2001. Vascular defense responses in rice: peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol. Plant Microbe Interact. 14:1411–1419 [DOI] [PubMed] [Google Scholar]
- 24. Hirayama O, Nakamura K, Hamada S, Kobayasi Y. 1994. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 29:149–150 [DOI] [PubMed] [Google Scholar]
- 25. Ito T. 1977. Toluidine blue—mode of photodynamic action in yeast cells. Photochem. Photobiol. 25:47–53 [DOI] [PubMed] [Google Scholar]
- 26. Johler S, Stephan R, Hartmann I, Kuehner KA, Lehner A. 2010. Genes involved in yellow pigmentation of Cronobacter sakazakii ES5 and influence of pigmentation on persistence and growth under environmental stress. Appl. Environ. Microbiol. 76:1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelley WL, Georgopoulos C. 1997. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc. Natl. Acad. Sci. U. S. A. 94:3679–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kovach ME, et al. 1995. Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 29. Krinsky NI. 1989. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 7:617–635 [DOI] [PubMed] [Google Scholar]
- 30. Krinsky NI. 2001. Carotenoids as antioxidants. Nutrition 17:815–817 [DOI] [PubMed] [Google Scholar]
- 31. Lamb C, Dixon RA. 1997. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:251–275 [DOI] [PubMed] [Google Scholar]
- 32. Liberati NT, et al. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. U. S. A. 103:2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu GY, et al. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 202:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu GY, Nizet V. 2009. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 17:406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohammadi M, Burbank L, Roper MC. 2012. Pantoea stewartii subsp. stewartii produces an endoglucanase that Is required for full virulence in sweet corn. Mol. Plant Microbe Interact. 25:463–470 [DOI] [PubMed] [Google Scholar]
- 36. Neudert U, Martinez-Ferez IM, Fraser PD, Sandmann G. 1998. Expression of an active phytoene synthase from Erwinia uredovora and biochemical properties of the enzyme. Biochim. Biophys. Acta 1392:51–58 [DOI] [PubMed] [Google Scholar]
- 37. Park YJ, et al. 2009. Virulence analysis and gene expression profiling of the pigment-deficient mutant of Xanthomonas oryzae pathovar oryzae. FEMS Microbiol. Lett. 301:149–155 [DOI] [PubMed] [Google Scholar]
- 38. Pericone CD, Park S, Imlay JA, Weiser JN. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 185:6815–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a. Perry KL, Simonitch TA, Harrison-Lavoie KJ, Liu ST. 1986. Cloning and regulation of Erwinia herbicola pigment genes. J. Bacteriol. 168:607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poplawsky AR, Urban SC, Chun W. 2000. Biological role of xanthomonadin pigments in Xanthomonas campestris pv. campestris. Appl. Environ. Microbiol. 66:5123–5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramakrishnan L, Tran HT, Federspiel NA, Falkow S. 1997. A crtB homolog essential for photochromogenicity in Mycobacterium marinum: isolation, characterization, and gene disruption via homologous recombination. J. Bacteriol. 179:5862–5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandmann G. 1994. Carotenoid biosynthesis in microorganisms and plants. Eur. J. Biochem. 223:7–24 [DOI] [PubMed] [Google Scholar]
- 42. Schaad NW, Jones JB, Chun W. 2001. Laboratory guide for identification of plant pathogenic bacteria, 3rd ed, p 177–178 APS Press, St. Paul, MN [Google Scholar]
- 43. Sedkova N, Tao L, Rouviere PE, Cheng Q. 2005. Diversity of carotenoid synthesis gene clusters from environmental Enterobacteriaceae strains. Appl. Environ. Microbiol. 71:8141–8146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a. Simon R, Priefer U, Pühler A. 1982. A broad host range mobilization system for in vivo genetics engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1:784–769 [Google Scholar]
- 44. Storz G, Imlay JA. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188–194 [DOI] [PubMed] [Google Scholar]
- 45. Tatsuzawa H, Maruyama T, Misawa N, Fujimori K, Nakano M. 2000. Quenching of singlet oxygen by carotenoids produced in Escherichia coli—attenuation of singlet oxygen-mediated bacterial killing by carotenoids. FEBS Lett. 484:280–284 [DOI] [PubMed] [Google Scholar]
- 46. To KY, et al. 1994. Analysis of the gene cluster encoding carotenoid biosynthesis in Erwinia herbicola Eho13. Microbiology 140:331–339 [DOI] [PubMed] [Google Scholar]
- 47. Tuveson RW, Larson RA, Kagan J. 1988. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J. Bacteriol. 170:4675–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Umeno D, Tobias AV, Arnold FH. 2005. Diversifying carotenoid biosynthetic pathways by directed evolution. Microbiol. Mol. Biol. Rev. 69:51–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Bodman SB, Bauer WD, Coplin DL. 2003. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 41:455–482 [DOI] [PubMed] [Google Scholar]
- 50. von Bodman SB, Majerczak DR, Coplin DL. 1998. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. U. S. A. 95:7687–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zang LY, Sommerburg O, van Kuijk FJ. 1997. Absorbance changes of carotenoids in different solvents. Free Radic. Biol. Med. 23:1086–1089 [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, et al. 2007. Knockout of crtB or crtI gene blocks the carotenoid biosynthetic pathway in Deinococcus radiodurans R1 and influences its resistance to oxidative DNA-damaging agents due to change of free radicals scavenging ability. Arch. Microbiol. 188:411–419 [DOI] [PubMed] [Google Scholar]