Abstract
Introduction:
Hedonic capacity is a dispositional ability to experience pleasure in response to stimuli that are typically rewarding. The ability to derive pleasure from natural reinforcers has been relatively overlooked as a risk factor for adolescent smoking. The present study sought to provide initial evidence for a relationship between hedonic capacity and adolescent smoking onset and escalation.
Methods:
The sample was composed of 1,106 adolescents participating in a prospective longitudinal survey study of adolescent health behaviors. Variables were measured via self-report every 6 months for 4 waves of data spanning 18 months. We hypothesized that adolescents with lower hedonic capacity may be less responsive to natural reinforcers and therefore be prone to take up and rely on smoking as a reinforcer.
Results:
A two-part latent growth curve model indicated that adolescents low in hedonic capacity were over two and a half times more likely to have smoked a cigarette in the past month at age 15.5 years (odds ratio = 2.64, 95% CI = 1.08–6.45) and to show a 90% increase (β = 0.9, z = 2.28, p = .02) in the rate of smoking escalation every 6 months across the following 18 months compared with adolescents with high hedonic capacity.
Conclusions:
This study provides the first evidence implicating hedonic capacity as a risk factor for adolescent smoking initiation and progression. Adolescents low in hedonic capacity may be an important population to target for smoking prevention and smoking cessation efforts possibly through behavioral skills to enhance pleasure derived through natural reinforcers.
Introduction
Over 20% of adolescents regularly smoke cigarettes (Centers for Disease Control and Prevention [CDC], 2008), with the percentage of regular smokers doubling and the percentage of daily smokers tripling from mid to late adolescence (CDC, 2010; Johnston, O’Malley, Bachman, & Schulenberg, 2008). Hedonic capacity is one trait that may explain individual differences in adolescent smoking uptake. Hedonic capacity is a heritable, stable dispositional ability to experience pleasure in response to stimuli that are typically rewarding (Bogdan & Pizzagalli, 2009; Meehl, 1987, 2001). It has received most attention as a hallmark characteristic of clinical depression (anhedonia), although it is relatively common for individuals to experience pleasure deficits without simultaneously experiencing other depression symptoms (Shafer, 2006; Watson, 2005).
Hedonic capacity is a continuous construct that varies considerably among the general population (Fawcett, Clark, Scheftner, & Hedeker, 1983; Harvey, Pruessner, Czechowska, & Lepage, 2007; Meehl, 1975, 1987). At one end of the continuum are individuals who find a broad array of life experiences as rewarding and experience a high degree of pleasure in response to rewards. At the other end of the continuum are individuals who do not affectively respond fully to typical rewarding experiences. It is thought that diminished responsiveness to typically rewarding stimuli reflects a disruption of neural pathways implicated in reward and motivation (Nestler & Carlezon, 2006; Tremblay et al., 2005). It is these individuals that may come to rely on unnatural or pharmacological rewards for pleasure, such as smoking, to stimulate an underresponsive reward system.
Although the ability to derive pleasure from natural reinforcers seems like a salient trait to consider in adolescent substance use, hedonic capacity has been relatively overlooked as a risk factor in adolescent smoking uptake. Since positive reinforcers are stimuli that increase the likelihood of behavior (Rescorla & Wagner, 1972), blunted responsiveness to natural reinforcers may lead to declines in those activities and a greater willingness to try pharmacological reinforcers, such as cigarettes. Thus, reduced hedonic capacity might predispose adolescents to smoke and to eventually become regular smokers. Until now, the role of hedonic capacity in smoking has only been examined in adults. Research in adult smokers has shown that lower hedonic capacity predicts greater cigarette cravings within 24 hr of smoking abstinence (Cook, Spring, McChargue, & Hedeker, 2004; Leventhal, Waters, Kahler, Ray, & Sussman, 2009), a tendency to place higher priority on smoking in comparison with other reinforcers (Leventhal et al., 2009), and a shorter time to smoking relapse (Cook, Spring, McChargue, & Doran, 2010). While low hedonic capacity may play a role in the maintenance of tobacco use among established smokers, little is known regarding whether this trait influences the initial uptake and progression of smoking, which typically occurs during adolescence. Investigating hedonic capacity in adolescents is important as adolescence is a high-risk period for smoking and the adolescent brain may be particularly responsive to drugs, such as nicotine that act on brain reward pathways and may alter hedonic tone.
The present study sought to provide initial evidence for a relationship between hedonic capacity and smoking onset and escalation among adolescents. We anticipated that low hedonic capacity would predict increased odds of smoking onset and increases in smoking rate across time. Smoking usually begins during adolescence, is carried well into adulthood, and is accompanied by significant morbidity and premature mortality (Eaton et al., 2006; Mokdad, Marks, Stroup, & Gerberding, 2004; Substance Abuse and Mental Health Services Administration, 2008). Identifying novel risk factors for smoking uptake is critical for understanding the bio-behavioral basis of adolescent smoking, early identification of youth at risk for smoking, and developing more effective smoking prevention and cessations programs.
Methods
Participants and Procedures
Participants were high school students (50% female and 74% White) taking part in a longitudinal study of the relationship between adolescent physical activity and adolescent smoking adoption. Participants were enrolled in one of four public high schools outside Philadelphia, PA, representing the gender, racial, ethnic, and socioeconomic characteristics of youth in the United States. The cohort was drawn from the 1,517 students identified through class rosters at the beginning of ninth grade. Students were ineligible to participate in this study if they had a special classroom placement (e.g., severe learning disability) or if they did not speak fluent English. Based on the selection criteria, a total of 1,487 (98%) students were eligible to participate. Of these 1,487 eligible teens, 1,478 (99%) had a parent’s consent to participate. Thirty adolescents were absent on the assent/survey days and 19 adolescents did not provide assent due to lack of interest in the study. Thus, 1,429 of 1,478 teens with parental consent (97%) provided their assent to participate and completed a baseline survey. A self-report 40-minute survey is administered every six months (fall and spring) on-site during compulsory classes each year of during high school.
The adolescent cohort was formed in the fall of 9th grade (Wave 1: 14 years of age) and is being followed until the spring of 12th grade (Wave 8: 18 years of age). The current analyses span four waves, beginning with Wave 4 when hedonic capacity was initially measured: Wave 4 (spring of 10th grade, N = 1,136), Wave 5 (fall of 11th grade), Wave 6 (spring of 11th grade), and Wave 7 (fall of 12th grade). Participants were individuals (N = 1,106) with complete data on the covariates. University Institutional Review Board approval of the study protocol was obtained.
Measures
Dependent Variable
Smoking was measured with a series of standard epidemiological questions regarding the number of days and the number of cigarettes smoked in the past month, such as “How many days in the past 30 days did you smoke a cigarette?” and “How many cigarettes did you smoke in the past 30 days?” (Eaton et al., 2006). Adolescents who reported never smoking or not smoking in the past thirty days received a zero for number of cigarettes smoked.
Independent Variable
Hedonic capacity was assessed with the 14-item Snaith–Hamilton Pleasure Scale (SHAPS). The SHAPS has excellent psychometric properties in nonclinical samples (Franken, Rassin, & Muris, 2007; Leventhal, Chasson, Tapia, Miller, & Pettit, 2006; Snaith et al., 1995). However, as the SHAPS has not yet been used with adolescents, we conducted an exploratory factor analysis to assess its factor structure. The results suggested a single-factor structure using both an Eigen values over 1 criterion and a visual inspection of the scree plot (plot of 14 Eigen values; Stevens, 2002). Correlations with the positive affect subscale (r = .30, p < .0001) and the depression subscale (r = −.20, p < .001) of the Center for Epidemiological Studies Depression (CES-D) inventory provide support for convergent and discriminant validity, respectively. Cronbach’s alpha in the current sample was r = .94. We summated the 14 items to generate a single hedonic capacity score with larger values indicating greater ability to subjectively experience pleasure to events that are typically rewarding. Response options ranged from definitely agree = 3 to definitely disagree = 0, with a possible range of 0–42. As in prior research of anhedonia and smoking (Cook, Spring, & McChargue, 2007), we used a median split to indicate higher (>34) versus low (≤34) hedonic capacity as the continuous measure was not normally distributed, with negative skew and positive kurtosis.
Covariates
The effects of several covariates important to smoking and to hedonic capacity (e.g., depression) were controlled for in the statistical model, including gender and race. All covariates were assessed at Wave 4, except demographics and impulsivity, which were measured at Wave 1.
Household smoking was assessed with a binary variable (0 = nobody living in the household smokes, 1 = at least one household member smokes). Peer smoking was measured by summating responses to three items asking whether the adolescent’s best friend smokes and whether and, if so, how many of his or her other four best male and four best female friends smoke (range 0–9 friends smoking; Audrain-McGovern, Rodriguez, & Kassel, 2009; Audrain-McGovern, Rodriguez, Tercyak, Neuner, & Moss, 2006).
Perceived parental monitoring was measured with a five-item Likert-style scale that evaluated adolescent perceptions of parental knowledge of whereabouts, activities, and friendships (DiClemente et al., 2001; Fletcher, Steinberg, & Williams-Wheeler, 2004; Kodl & Mermelstein, 2004; Simons-Morton, 2004). Response options range from 1 = almost nothing to 3 = a lot. Higher scores indicated greater monitoring (Cronbach’s α = .82). Depression symptoms were assessed with the CES-D inventory. The CES-D is a valid and reliable 20-item self-report measure designed to assess depression symptoms in the general population (Radloff, 1977, 1991; Roberts, Andrews, Lewinsohn, & Hops, 1990). Scores range from 0 to 60, with scores above 22 in adolescents indicative of a clinical level of depressive symptoms. Impulsivity was measured with the impulsivity subscale of the Temperament & Character Inventory (TCI; 5 True/False items; Cloninger, Przybeck, Svrakic, & Wetzel, 1994). Impulsivity and similar constructs as measured by the TCI, and its predecessor the Temperament Personality Questionnaire, have been linked to adolescent smoking and substance use (Audrain-McGovern et al., 2004; Wills, Vaccaro, & McNamara, 1994; Wills, Windle, & Cleary, 1998).
Data Analysis
Univariate statistics were generated to describe the study population in terms of demographics and smoking. Univariate estimates were generated with SAS v. 9.1.3 software.
Latent Growth Modeling
A latent growth modeling (LGCM) was conducted to assess the effects of hedonic capacity on smoking. LGCM is a structural equation modeling method that models repeated observed measures (measured variables) on factors (latent variables) representing random effects (ηs; Duncan & Duncan, 1995). A level factor is used to represent baseline and trend factors are used to represent growth or rate of change across time (i.e., each unit change in time is associated with a η change in a given process).
In the present analysis, we conducted a two-part LGCM (Olsen & Schafer, 2001). The two-part LGCM method is ideal for modeling count variables with a preponderance of zeros (zero inflated), such as the number of cigarettes smoked in the past thirty days in a community sample of adolescents. As there are more adolescent nonsmokers than smokers, there will be a preponderance of zeros in the number of cigarettes smoked per month. However, instead of eliminating nonsmokers, the two-part model permits the inclusion of two correlated latent growth curves, one for initiation of use (modeling transition from nonsmoking to smoking) and one for change in the number of cigarettes smoked per month among smokers. As such, the two-part model includes a binary part for modeling smoking versus nonsmoking and a continuous part for modeling change in the number of cigarettes smoked among adolescents who reported previous smoking. If an adolescent has not smoked in the past thirty days, they receive a zero for the binary part of the model and do not contribute to the continuous part of the model; the individual’s data are treated as if it were missing data in the continuous part. If the adolescent reports smoking within the past thirty days at a given wave, the adolescent’s data are added to the continuous portion of the model. LGCM was conducted using Mplus v. 6.0 software (Muthén & Muthén, 1998–2010).
Missing Data
Because hedonic capacity was not measured until Wave 4 (N = 1,136), we used Wave 4 (age 15–16 years) as the baseline for these analyses. Due to wave nonresponse and loss to follow-up, the number of adolescents who completed a survey in the subsequent waves were 1,110 (Wave 5), 1,092 (Wave 6), and 1,090 (Wave 7). Thirty adolescents had missing data on the covariates at Wave 4 and were not included in the analysis. To account for 14–16 cases with missing smoking data at Waves 6 and 7, multivariate modeling used all available data. Mplus allows modeling with missing data using maximum likelihood estimation of the mean, variance, and covariance parameters, employing the expectation maximization algorithm when data are missing at random (Muthén & Muthén, 1998–2004). Final analyses were based on 1,106 adolescents.
Results
Descriptive Statistics
Table 1 presents the means and SDs for continuous model variables, along with the proportions for the categorical model variables. Cross-sectional tabulations for cigarette smoking in the past thirty days indicated that the proportion of adolescents who did not smoke in the past month decreased from 89% at Wave 4 baseline to 87% at Wave 7. At the same time, the mean number of cigarettes smoked among those adolescents smoking at least one cigarette in the past thirty days increased from 3.43 (SD = 3.64) at Wave 4 baseline to 3.67 (SD = 3.81) cigarettes at Wave 7. The average hedonic capacity score for the sample was 33.03 (SD = 8.32), which is very similar to mean scores in previous samples of undergraduates (M = 33.6 in Franken et al., 2007; M = 34.4 in Leventhal et al., 2006). Table 2 provides correlations between hedonic capacity and the study variables.
Table 1.
Characteristics of the Sample
| Variable | Level | N | % |
| Gender | Female | 566 | 51 |
| Male | 540 | 49 | |
| Race | Black | 145 | 13 |
| White | 833 | 75 | |
| Other race | 128 | 12 | |
| Household smoking | At least one member smokes | 412 | 37 |
| No household members smoke | 694 | 63 | |
| Hedonic capacity | Low (≤34) | 561 | 51 |
| High (>34) | 545 | 49 | |
| Binary smoking, spring 10th grade | Smoked 0 cigarettes in the past 30 days | 962 | 89 |
| Smoked ≥1 cigarettes in the past 30 days | 120 | 11 | |
| Binary smoking, fall 11th grade | Smoked 0 cigarettes in the past 30 days | 822 | 88 |
| Smoked ≥1 cigarettes in the past 30 days | 108 | 12 | |
| Binary smoking, spring 11th grade | Smoked 0 cigarettes in the past 30 days | 848 | 90 |
| Smoked ≥1 cigarettes in the past 30 days | 98 | 10 | |
| Binary smoking, fall 12th grade | Smoked 0 cigarettes in the past 30 days | 841 | 87 |
| Smoked ≥1 cigarettes in the past 30 days | 120 | 13 | |
| Mean | SD | ||
| Age | 15.70 | 0.56 | |
| Impulsivity | 7.99 | 1.43 | |
| Depressive symptoms | 15.54 | 10.42 | |
| Parental monitoring | 11.98 | 2.83 | |
| Peers smoking (0–9 peers) | 1.77 | 2.50 | |
| Cigarettes smoked, spring 10th grade | 3.43 | 3.64 | |
| Cigarettes smoked, fall 11th grade | 3.22 | 3.39 | |
| Cigarettes smoked, spring 11th grade | 3.86 | 3.18 | |
| Cigarettes smoked, fall 12th grade | 3.67 | 3.81 |
Table 2.
Correlations Between Hedonic Capacity and the Study Variables
| Variable | Correlation | p Value |
| Female | .27 | <.05 |
| Black | .02 | >.05 |
| Other | .20 | <.05 |
| Household smoking | −.11 | <.05 |
| Age | −.04 | >.05 |
| Peer smoking | −.05 | >.05 |
| Impulsivity | −.11 | <.01 |
| Parental monitoring | .15 | <.0001 |
| Depression symptoms | −.09 | <.01 |
| Number of cigarettes/30 days (spring 10) | −.09 | <.01 |
| Number of cigarettes/30 days (fall 11) | −.11 | <.01 |
| Number of cigarettes/30 days (spring 11) | −.13 | <.01 |
| Number of cigarettes/30 days (fall 12) | −.12 | <.01 |
Note. Biserial correlations are reported for the continuous variables and tetrachoric correlations are reported for binary variables.
Multivariate Model
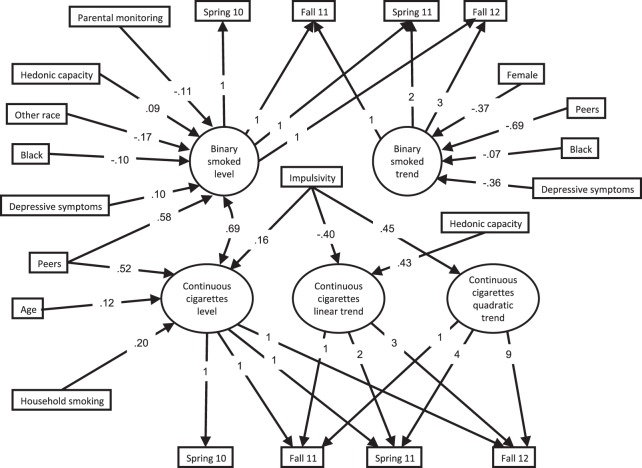
We began by assessing the binary and continuous conditional models separately for fit to the data (i.e., two separate models). This permitted identifying the average trajectory shape for each part. For the binary part, a linear growth curve fit the data well, , p = .20, comparative fit index (CFI) = 1.00, root mean squared error of approximation (RMSEA) = 0.01, Weighted Root Mean Square Residual = 0.54. We next assessed the continuous model, modeling only data from participants smoking at least one cigarette in the past thirty days. A visual inspection of the observed mean plot suggested a quadratic trend. Indeed, although the linear model fit the data well, , p = .43, CFI = 1.00, RMSEA = 0.03, 95% CI = 0–0.07, Standardized Root Mean Square Residual (SRMR) = 0.04, the addition of a quadratic trend resulted in a significantly better model fit, , p = .02. The continuous model with a quadratic trend fit the data well, , p = .85, CFI = 1.00, RMSEA = 0.00, 95% CI = 0–0.04, SRMR = 0.02. As such, our two-part model included a binary linear growth part along with a quadratic continuous part. A graphic representation of the two-part model is presented in Figure 1 with standardized regression coefficients for significant paths only. Table 3 provides the statistics for the model regressions.
Figure 1.
The effects of hedonic capacity on the number of cigarettes smoked over time (spring Grade 10 through fall Grade 12). Note: Only significant paths with standardized path coefficients are shown in the model.
Table 3.
Unstandardized Regression Coefficients, Standard Errors, z-Statistics, and p Values for All Model Regressions
| Binary smoked level | Binary smoked trend | |||||||||||
| β | SE | z | p Value | β | SE | z | p Value | |||||
| Hedonic capacity | 0.97 | 0.46 | 2.13 | .03 | −0.04 | 0.17 | −0.26 | .80 | ||||
| Age | 0.12 | 0.14 | 0.88 | .38 | −0.06 | 0.13 | −0.43 | .67 | ||||
| Female | 0.15 | 0.46 | 0.33 | .74 | −0.36 | 0.17 | −2.08 | .04 | ||||
| Black race | −1.54 | 0.76 | −2.03 | .04 | −0.10 | 0.31 | −0.33 | .74 | ||||
| Other race | −2.78 | 1.03 | −2.71 | .01 | −0.08 | 0.42 | −0.18 | .86 | ||||
| Household smoking | 0.80 | 0.47 | 1.70 | .09 | 0.42 | 0.18 | 2.40 | .02 | ||||
| Peers smoking | 1.20 | 0.11 | 10.70 | .00 | −0.13 | 0.04 | −3.79 | .00 | ||||
| Impulsivity | 0.22 | 0.16 | 1.37 | .17 | 0.07 | 0.06 | 1.17 | .24 | ||||
| Parental monitoring | −0.21 | 0.07 | −2.79 | .01 | 0.00 | 0.03 | 0.08 | .94 | ||||
| Depressive symptoms | 0.05 | 0.02 | 2.47 | .01 | −0.02 | 0.01 | −2.17 | .03 | ||||
| Continuous cigarettes level | Continuous cigarettes linear trend | Continuous cigarettes quadratic trend | ||||||||||
| β | SE | z | p Value | β | SE | z | p Value | β | SE | z | p Value | |
| Hedonic capacity | 0.11 | 0.29 | 0.38 | .71 | 0.90 | 0.40 | 2.28 | .02 | −0.24 | 0.12 | −1.94 | .05 |
| Age | 0.54 | 0.21 | 2.53 | .01 | −0.21 | 0.30 | −0.70 | .49 | 0.06 | 0.10 | 0.63 | .53 |
| Female | −0.58 | 0.31 | −1.88 | .06 | 0.12 | 0.36 | 0.33 | .74 | −0.02 | 0.11 | −0.17 | .86 |
| Black race | −0.82 | 0.55 | −1.48 | .14 | −1.46 | 0.82 | −1.79 | .07 | 0.38 | 0.29 | 1.33 | .18 |
| Other race | −0.77 | 0.45 | −1.73 | .08 | −1.44 | 1.04 | −1.38 | .17 | 0.49 | 0.39 | 1.27 | .20 |
| Household smoking | 1.02 | 0.31 | 3.26 | .00 | −0.61 | 0.38 | −1.62 | .11 | 0.23 | 0.12 | 1.93 | .05 |
| Peers smoking | 0.50 | 0.07 | 7.54 | .00 | −0.13 | 0.07 | −1.88 | .06 | 0.02 | 0.02 | 1.17 | .24 |
| Impulsivity | 0.27 | 0.10 | 2.63 | .01 | −0.29 | 0.12 | −2.42 | .02 | 0.09 | 0.04 | 2.37 | .02 |
| Parental monitoring | −0.07 | 0.05 | −1.43 | .15 | 0.06 | 0.07 | 0.83 | .41 | −0.01 | 0.02 | −0.57 | .57 |
| Depressive symptoms | 0.01 | 0.01 | 0.43 | .67 | 0.03 | 0.02 | 1.61 | .11 | −0.01 | 0.01 | −1.18 | .24 |
Note. Female (1 = yes, 0 = male); Black (1 = yes, 0 = else); other race (other race = 1, else = 0); household smoking (0 = nobody living in the household smokes, 1 = at least one person living in the household smokes); peers smoking (number of peers smoking, 0–9 peers).
Effect of Hedonic Capacity on Whether an Adolescent Smoked in the Past Month (Binary Model)
Baseline Level
Compared with participants with high hedonic capacity, participants low in hedonic capacity were over two and a half times (odds ratio [OR] = 2.64, 95% CI = 1.08–6.45) more likely to have smoked a cigarette in the past thirty days. Peers smoking (OR = 3.30, 95% CI = 2.65–4.12) and depressive symptoms (OR = 1.05, 95% CI = 1.01–1.09) were also associated with an increased risk of smoking a cigarette in the past thirty days. In contrast, an SD (= 2.83) increase in parental monitoring was associated with a 46% reduction (OR = 0.54, 95% CI = 0.36–0.82) in the odds of smoking a cigarette in the past thirty days. Being Black (OR = 0.21, 95% CI = 0.05–0.95) or another race (OR = 0.06, 95% CI = 0.01–0.47) was also associated with a decreased likelihood of smoking a cigarette in the past thirty days compared with being White.
Linear Trend
While those low in hedonic capacity were more likely to have smoked a cigarette in the past thirty days at baseline compared with adolescents high in hedonic capacity, low hedonic capacity did not significantly increase the odds of smoking a cigarette in the past month at subsequent time points. Being female (OR = 0.70, 95% CI = 0.50–0.98) and peer smoking (OR = 0.88, 95% CI = 0.82–0.94) were both associated with a decreased rate of acceleration in the likelihood of smoking a cigarette in the past thirty days for each six month increase in time. In contrast, having at least one household member whom smokes was associated with a 53% increase (OR = 1.53, 95% CI = 1.08–2.16) in the odds of progressing to smoking a cigarette for each six-month increase in time.
Effect of Hedonic Capacity on the Number of Cigarettes Smoked in the Past Month (Continuous Model)
Baseline Level
Hedonic capacity was not significantly associated with number of cigarettes smoked in the past month at baseline (i.e., no significant path to the level factor). Impulsivity had a significant positive effect on the number of cigarettes smoked at baseline. More specifically, a unit increase in impulsivity was associated with a .27 increase (β = 0.27, z = 2.63, p = .01) in log cigarettes smoked in the past thirty days. Since the number of cigarettes smoked was log transformed, this can be translated into a .27 × 100 or a 27% change in the number of cigarettes smoked for each one-point increase in impulsivity. Other factors associated with an increase in the number of cigarettes smoked in the past thirty days were age (β = 0.54, z = 2.53, p = .01), household smoking (β = 1.02, z = 3.26, p < .0001), and peers smoking (β = 0.50, z = 7.54, p < .0001).
Linear Trend
Low hedonic capacity was associated with .9 increase (β = 0.9, z = 2.28, p = .02) in the rate of change in the log cigarettes smoked in the past thirty days for each six-month increase in time. This can be translated to a 90% increase in the rate of change for participants with low compared with high hedonic capacity. Impulsivity, in contrast was associated with a decreased acceleration in the number of cigarettes smoked in the past thirty days (β = −0.29, z = −2.42, p = .02).
Continuous Cigarettes Smoked Quadratic Trend
Impulsivity was the only covariate to have a significant effect on the quadratic trend. Impulsivity was associated with an increased deceleration from baseline (β = 0.09, z = 2.37, p = .02). A marginally significant effect was observed for hedonic capacity. Lower hedonic capacity was associated with a significantly decreased deceleration in the number of cigarettes smoked in the past thirty days (β = −0.24, z = −1.94, p = .05). This can be translated into a 24% decrease in the rate of deceleration for low compared with high hedonic capacity, holding all other independent variables constant. Thus, smoking rate was not leveling off for the adolescents with low hedonic capacity compared with those with hedonic capacity.
Discussion
The present study provides the first evidence implicating hedonic capacity as a risk factor for adolescent smoking initiation and progression. We hypothesized that adolescents with lower hedonic capacity may be less responsive to natural reinforcers and therefore be prone to take up and rely on smoking as a reinforcer. Indeed, the results indicated that adolescents low in hedonic capacity were over two and a half times more likely to have smoked a cigarette in the past month at age 15 and to show a 90% increase in the rate of smoking escalation across the following 18 months compared with adolescents with high hedonic capacity.
These findings extend past results implicating low hedonic capacity in the maintenance of smoking in adults (Cook et al., 2004; Cook et al., 2010; Leventhal et al., 2009) and suggest that this trait is a marker for a preexisting vulnerability for the early progression of smoking. One possibility is that low hedonic capacity may confer risk for the likely use of pharmacological reinforcers, and the findings involving smoking in the current study may be characteristic of general propensity toward uptake of a variety of nonpharmacological reinforcers. Indeed, other studies have demonstrated associations between anhedonia and use of other drugs, including cocaine and amphetamine (Leventhal et al., 2008, 2010), cannabis (Bonn-Miller, Zvolensky, Marshall, & Bernstein, 2007; Dorard, Berthoz, Phan, Corcos, & Bungener, 2008; Dumas et al., 2002; Johnson, Bonn-Miller, Leyro, & Zvolensky, 2009), and opiates (Zijlstra, Veltman, Booij, van den Brink, & Franken, 2009). Regardless of whether low hedonic capacity may confer risk to other drugs of abuse, the current study suggests that this trait may be an important variable to identify youth at risk for smoking.
Adolescence is a critical period for the development of neural systems that regulate reward processing (Forbes & Dahl, 2005; Galvan, 2010). A reduced ability to experience pleasure is associated with diminished mesocorticolimbic dopaminergic activity (Stein, 2008; Treadway & Zald, 2011; Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002; Tremblay et al., 2005) and sensitivity to the effects of nondrug reinforcers on phasic mesocorticolimbic dopamine release (Juckel et al., 2006). Decreased dopamine release may create a neurobiological context for increased sensitivity to drugs that stimulate the mesocorticolimbic pathway (Tremblay et al., 2002). Nicotine stimulates dopamine neurotransmission and other brain systems associated with reward, which might help anhedonic adolescents overcome pleasure deficits (Barrett, Boileau, Okker, Pihl, & Dagher, 2004; Brody et al., 2004, 2009; Epping-Jordan, Watkins, Koob, & Markou, 1998). However, long-term chronic exposure to nicotine may further reduce reward functioning. It is possible that adolescents who began smoking as a result of low hedonic capacity may, after years of chronic nicotine exposure, further elevate their reward threshold (Koob & Le Moal, 2008). This suggests that smoking to regulate reward deficits may lead to even greater need to smoke.
While research suggests that cigarettes alone only produce a mild increment in pleasurable emotions, there is evidence, however, that nicotine may augment pleasure response to nondrug stimuli (e.g., secondary reinforcing effects of smoking; Caggiula et al., 2009; Chaudhri et al., 2006). We believe that adolescents with low hedonic capacity may be particularly sensitive to the secondary (reward-enhancing) effects of nicotine, which may account for these findings. That is, adolescents with lower hedonic capacity smoke during appetitive events in order to increase their pleasurable response to such events. Preclinical models suggest that nicotine increases reward sensitivity or the pleasure derived from available reinforcers in the environment (Kenny & Markou, 2006). Thus, adolescents may self-administer nicotine to pharmacologically stimulate an underresponsive reward system. Nicotine may allow adolescents to increase their hedonic tone and derive more pleasure from natural reinforcers in their environment. This explanation is consistent with the finding of Cook et al. (2007) that adults with low (vs. high) hedonic capacity are more sensitive to the affect-enhancing effects of a nonpharmacological positive mood induction only after nicotine administration.
Clinically, these findings suggest that adolescents low in hedonic capacity may be an important population to target for smoking prevention and smoking cessation efforts. However, finding suitable alternative reinforcers may be more difficult when we consider that adolescents with low hedonic capacity may underrespond to typical alternative rewards. Activities that are rewarding for those with normal levels of hedonic capacity may not be as rewarding for those with low levels. If smoking’s role is to permit an adolescent to derive greater reward from natural reinforcers, the more important question may be how to enhance pleasure from typical reinforcers rather than identifying reinforcers potent enough to elicit feelings of pleasure. Drawing from Positive Psychology approaches to the treatment of depression, behavioral skills to magnify or savor the enjoyment derived from daily reinforcers may help ameliorate the pleasure deficit associated with lower hedonic capacity (Lee Duckworth, Steen, & Seligman, 2005; Seligman, Steen, Park, & Peterson, 2005). However, the present findings need to be replicated before ultimately determining their clinical value.
To our knowledge, this is the first investigation of whether hedonic capacity influences adolescent smoking onset and escalation. As such, the study has several strengths including a large sample of adolescents, four measurement waves across 18 months, and control for potential confounding variables (e.g., depression) in the statistical model. One potential limitation is that the hedonic capacity measure used in this study, the SHAPS, has not yet been used with adolescents. However, consistent with adult populations, the factor analysis indicated a single factor structure. The average hedonic capacity score in the present study is comparable with that reported for young adults (e.g., 34; Leventhal et al., 2009). In this community sample of adolescents, we were able to detect a level at which adolescents were at risk for smoking, despite controlling for depression. Conceptually, the finding that anhedonia predicted smoking uptake over and above depression suggest that anhedonia is a risk factor for smoking simply because it is a marker for experiencing depressive symptoms in gender. Perhaps, anhedonia may reflect a subtype of depressive pathology that confers greater risk of smoking uptake than other facets of depression. To test this hypothesis, future research should directly compare the relative predictive validity of anhedonia versus other facets of depression (e.g., somatic and neurovegetative features, negative affect, interpersonal problems) in explaining variation in risk for smoking uptake.
In addition, we relied on self-reports of smoking as biochemical verification of smoking status are not typically implemented in epidemiological studies as adolescent self-reports have been determined to be valid and sufficient, especially when confidentiality is assured (Velicer, Prochaska, Rossi, & Snow, 1992; Wills & Cleary, 1997). Furthermore, the standard cotinine cutoff of 15 ng/ml cannot validate cotinine levels consistent with definitions of being an adolescent current smoker (e.g., one cigarette in the past thirty days; Dolcini, Adler, Lee, & Bauman, 2003). Finally, the present study did not examine mechanisms that link hedonic capacity to smoking. Though we speculate that increased sensitivity to nicotine’s secondary reinforcing effects is a putative mediator, a greater understanding of the bio-behavioral mechanisms than underpin hedonic capacity’s link to smoking may help inform novel interventions to reduce adolescent smoking prevalence.
In summary, the present study provides support for hedonic capacity as a novel determinant of adolescent smoking uptake. Low hedonic capacity predicted initiating smoking by age 16 and the escalation in the number of cigarettes smoked across the following 18 months. Thus, hedonic capacity appears to predict earlier smoking onset and subsequent escalation. As such, it may be a useful variable to identify youth at risk for smoking over and above other affective characteristics, such as depression. More research is warranted to determine the processes by which hedonic capacity influences smoking to inform interventions to prevent smoking uptake and facilitate smoking cessation in those already smoking.
Funding
This study was supported by the National Cancer Institute RO1 CA126958 (JAM) and National Institute on Drug Abuse K08 DA025041 (AML).
Declaration of Interests
None declared.
References
- Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: Evidence for self-medication and peer smoking mediation. Addiction. 2009;104:1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. doi:10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Cuevas J, Rodgers K, Patterson F. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:2023–2034. Retrieved from http://cebp.aacrjournals.org/content/13/12/2023.full. [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Neuner G, Moss HB. The impact of self-control indices on peer smoking and adolescent smoking progression. Journal of Pediatric Psychology. 2006;31:139–151. doi: 10.1093/jpepsy/jsi079. doi:10.1093/jpepsy/jsi079. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54:65–71. doi: 10.1002/syn.20066. doi:10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. The heritability of hedonic capacity and perceived stress: A twin study evaluation of candidate depressive phenotypes. Psychological Medicine. 2009;39:211–218. doi: 10.1017/S0033291708003619. doi:10.1017/S0033291708003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ, Marshall EC, Bernstein A. Incremental validity of anxiety sensitivity in relation to marijuana withdrawal symptoms. Addictive Behaviors. 2007;32:1843–1851. doi: 10.1016/j.addbeh.2006.12.016. doi:10.1016/j.addbeh.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, Abrams AL, Costello MR, Khan A, Kozman D, et al. Effect of a history of major depressive disorder on smoking-induced dopamine release. Biological Psychiatry. 2009;66:898–901. doi: 10.1016/j.biopsych.2009.06.011. doi:10.1016/j.biopsych.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. doi:10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: A dual-reinforcement model. Nebraska Symposium on Motivation. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. doi:10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. Morbidity & Mortality Weekly Report. 2008;57:1226–1228. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a3.htm. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette use among high school students—United States, 1991–2009. Morbidity & Mortality Weekly Report. 2010;59:797–801. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5926a1.htm. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. doi:10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The temperament and character inventory (TCI): A guide to its development and use. St. Louis, MO: Center for Psychobiology and Personality; 1994. [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine & Tobacco Research. 2010;12:978–982. doi: 10.1093/ntr/ntq118. doi:10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. doi:10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. doi:10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- DiClemente RJ, Wingood GM, Crosby R, Sionean C, Cobb BK, Harrington K, et al. Parental monitoring: Association with adolescents’ risk behaviors. Pediatrics. 2001;107:1363–1368. doi: 10.1542/peds.107.6.1363. doi:10.1542/peds.107.6.1363. [DOI] [PubMed] [Google Scholar]
- Dolcini MM, Adler NE, Lee P, Bauman KE. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine & Tobacco Research. 2003;5:473–483. doi:10.1080/1462220031000118586. [PubMed] [Google Scholar]
- Dorard G, Berthoz S, Phan O, Corcos M, Bungener C. Affect dysregulation in cannabis abusers: A study in adolescents and young adults. European Child & Adolescent Psychiatry. 2008;17:274–282. doi: 10.1007/s00787-007-0663-7. doi:10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- Dumas P, Saoud M, Bouafia S, Gutknecht C, Ecochard R, Dalery J, et al. Cannabis use correlates with schizotypal personality traits in healthy students. Psychiatry Research. 2002;109:27–35. doi: 10.1016/s0165-1781(01)00358-4. doi:10.1016/S0165-1781(01)00358-4. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC. Modelling the process of development via latent variable growth curve methodology. Structural Equation Modeling. 1995;2:187–213. doi:10.1080/10705519509540009. [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, et al. Youth risk behavior surveillance—United States, 2005. Morbidity & Mortality Weekly Report Surveillance Summaries. 2006;55:1–108. doi:10.1111/j.1746-1561.2006.00127.x. [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. doi:10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Hedeker D. Differences between anhedonic and normally hedonic depressive states. American Journal of Psychiatry. 1983;140:1027–1030. doi: 10.1176/ajp.140.8.1027. Retrieved from http://ajp.psychiatryonline.org/journal.aspx?journalid=13. [DOI] [PubMed] [Google Scholar]
- Fletcher AC, Steinberg L, Williams-Wheeler M. Parental influences on adolescent problem behavior: Revisiting Stattin and Kerr. Child Development. 2004;75:781–796. doi: 10.1111/j.1467-8624.2004.00706.x. doi:10.1111/j.1467-8624.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. doi:10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of Affective Disorders. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. doi:10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. doi:10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non-clinical subjects. Molecular Psychiatry. 2007;12 doi: 10.1038/sj.mp.4002021. 703, 767–775. doi:10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Bonn-Miller MO, Leyro TM, Zvolensky MJ. Anxious arousal and anhedonic depression symptoms and the frequency of current marijuana use: Testing the mediating role of marijuana-use coping motives among active users. Journal of Studies on Alcohol and Drugs. 2009;70:543–550. doi: 10.15288/jsad.2009.70.543. Retrieved from http://www.jsad.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2007: Volume I, secondary school students. 2008. Bethesda, MD: National Institute on Drug Abuse. (NIH Publication No. 08-6418A) [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. doi:10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. doi:10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kodl MM, Mermelstein R. Beyond modeling: Parenting practices, parental smoking history, and adolescent cigarette smoking. Addictive Behaviors. 2004;29:17–32. doi: 10.1016/s0306-4603(03)00087-x. doi:10.1016/S0306-4603(03)00087-X. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. doi:10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lee Duckworth A, Steen TA, Seligman ME. Positive psychology in clinical practice. Annual Review of Clinical Psychology. 2005;1:629–651. doi: 10.1146/annurev.clinpsy.1.102803.144154. doi:10.1146/annurev.clinpsy.1.102803.144154. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greenberg J, Mickens L, Ray LA, et al. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Experimental and Clinical Psychopharmacology. 2010;18:562–569. doi: 10.1037/a0021964. doi:10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: A psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62:1545–1558. doi: 10.1002/jclp.20327. doi:10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, et al. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2008;17:218–223. doi: 10.1080/10550490802019774. doi:10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine & Tobacco Research. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. doi:10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: Some conjectures. Bulletin of The Menninger Clinic. 1975;39:295–307. Retrieved from http://www.tc.umn.edu/~pemeehl/106HedonicCapacity.pdf. [PubMed] [Google Scholar]
- Meehl PE. “Hedonic capacity” ten years later: Some clarifications. In: Clark DC, Fawcett J, editors. Anhedonia and affect deficit states. New York: PMA Publishing; 1987. pp. 47–50. [Google Scholar]
- Meehl PE. Primary and secondary hypohedonia. Journal of Abnormal Psychology. 2001;110:188–193. doi: 10.1037//0021-843x.110.1.188. doi:10.1037//0021-843X.110.1.188. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. doi:10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 3rd ed. Los Angeles, CA: Muthén & Muthén; 1998–2004. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. doi:10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Olsen MK, Schafer JL. A two-part random effects model for semicontinuous longitudinal data. Journal of the American Statistical Association. 2001;96:730–745. doi:10.1198/016214501753168389. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Radloff LS. The use of the center for epidemiologic studies depression scale in adolescents and young adults. Journal of Youth and Adolescence. 1991;20(2):149–166. doi: 10.1007/BF01537606. doi:10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Roberts RE, Andrews JA, Lewinsohn PM, Hops H. Assessment of depression in adolescents using the center for epidemiologic studies depression scale. Psychological Assessment. 1990;2:122–128. doi:10.1037/1040-3590.2.2.122. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health, NSDUH: Detailed Tables. HHS; 2008. Retrieved from http://www.oas.samhsa.gov/NSDUH/2k7NSDUH/tabs/Sect4peTabs10to11.pdf. [Google Scholar]
- Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: Empirical validation of interventions. American Psychologist. 2005;60:410–421. doi: 10.1037/0003-066X.60.5.410. doi:10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62:123–146. doi: 10.1002/jclp.20213. doi:10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Simons-Morton BG. The protective effect of parental expectations against early adolescent smoking initiation. Health Education Research. 2004;19:561–569. doi: 10.1093/her/cyg071. doi:10.1093/her/cyg071. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. doi:10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: The role of dopaminergic neurocircuitry. CNS Spectrums. 2008;13:561–565. doi: 10.1017/s1092852900016837. Retrieved from http://www.cnsspectrums.com/aspx/issue.aspx?issue=0. [DOI] [PubMed] [Google Scholar]
- Stevens JP. Applied multivariate statistics for the social sciences. 4th ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. doi:10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: Altered response to dextroamphetamine. Archives of General Psychiatry. 2002;59:409–416. doi: 10.1001/archpsyc.59.5.409. doi:10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. doi:10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychological Bulletin. 1992;111:23–41. doi: 10.1037/0033-2909.111.1.23. doi:10.1037//0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. doi:10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Wills TA, Cleary SD. The validity of self-reports of smoking: Analyses by race/ethnicity in a school sample of urban adolescents. American Journal of Public Health. 1997;87:56–61. doi: 10.2105/ajph.87.1.56. doi:10.2105/AJPH.87.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: An application of Cloninger’s theory. Journal of Substance Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. doi:10.1016/S0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Wills TA, Windle M, Cleary SD. Temperament and novelty seeking in adolescent substance use: Convergence of dimensions of temperament with constructs from Cloninger’s theory. Journal of Personality and Social Psychology. 1998;74:387–406. doi: 10.1037//0022-3514.74.2.387. doi:10.1037/0022-3514.74.2.387. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug and Alcohol Dependence. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. doi:10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]