Macrophage production of CXCL10 amplifies the production of IL-6 by B cells, leading to plasma cell differentiation.
Abstract
In tonsils, CD138+ plasma cells (PCs) are surrounded by CD163+ resident macrophages (Mϕs). We show here that human Mϕs (isolated from tonsils or generated from monocytes in vitro) drive activated B cells to differentiate into CD138+CD38++ PCs through secreted CXCL10/IP-10 and VCAM-1 contact. IP-10 production by Mϕs is induced by B cell–derived IL-6 and depends on STAT3 phosphorylation. Furthermore, IP-10 amplifies the production of IL-6 by B cells, which sustains the STAT3 signals that lead to PC differentiation. IP-10–deficient mice challenged with NP-Ficoll show a decreased frequency of NP-specific PCs and lower titers of antibodies. Thus, our results reveal a novel dialog between Mϕs and B cells, in which IP-10 acts as a PC differentiation factor.
Plasma cells (PCs) include short-lived PCs found in the extrafollicular foci of peripheral lymphoid organs and long-lived PCs in the bone marrow. Short-lived PCs account for the initial immune response after antigen (Ag) encounter, whereas long-lived PCs can produce antibodies (Abs) for years and thus provide life-long protection (Kunkel and Butcher, 2003; Shapiro-Shelef and Calame, 2005; Radbruch et al., 2006; Hiepe et al., 2011). Our understanding of the mechanisms underlying the rapid formation of Ab-secreting PCs during early B cell responses remains incomplete (Oracki et al., 2010). B cell activation is initiated after engagement of the BCR by a specific Ag in both T cell–dependent (TD) and T cell–independent (TI) manners (Mond et al., 1995; Fagarasan and Honjo, 2000). Most long-lived PCs in the bone marrow are derived from TD responses involving germinal center reactions. Interestingly, large numbers of PCs and plasmablasts generated in both TD and TI responses die within a few days of being produced (Mond et al., 1995; Smith et al., 1996; García de Vinuesa, 1999; Shapiro-Shelef and Calame, 2005). However, emerging evidence indicates that long-lived Ab responses can also be induced by some TI challenges (Alugupalli et al., 2004; Hsu et al., 2006; Obukhanych and Nussenzweig, 2006). The TI response is critical for the host to provide prompt protection against invading pathogens and their products, such as viral glycoproteins and bacterial polysaccharides which stimulate IgG and IgA production in the absence of CD40L signals (Mond et al., 1995). One of the APCs, DCs, could also induce TI class switching through the secretion of BLyS and APRIL (Litinskiy et al., 2002).
To maximize the probability of mounting a rapid and appropriate response, B cells encounter Ags in lymphoid organs, including lymph nodes, spleen, Peyer’s patches, and tonsils (Batista and Harwood, 2009). In lymph nodes, large Ags such as particulates, immune complexes, and viruses that travel through the subcapsular sinus are picked up by resident macrophages (Mϕs) and presented to follicular B cells (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2009). The recognition of Ags on the surfaces of APCs by B cells results in efficient formation of an immune synapse (Harwood and Batista, 2010; Pierce and Liu, 2010), which has been proven to be much more active and dynamic than that predicted from the response of B cells to Ags in solution (Fleire et al., 2006). The question remains whether APCs also provide “second” signals directing differentiation of PCs, in addition to the initiation of BCR signaling.
After engagement of BCR signaling, the differentiation process of B cells into PCs depends on a combination of signals, including Ags, soluble mediators (such as IL-2, IL-3, IL-4, IL-6, IL-10, IL-15, IL-21, IFN-α, TNF, BAFF, and APRIL), pathogen-associated molecule patterns, and signals from T cells and APCs (Fairfax et al., 2008). Terminally differentiated PCs are quiescent cells that express CD138 (syndecan-1; Chilosi et al., 1999; Medina et al., 2002). Unlike the generation of CD138−CD38++CD20− plasmablasts, the mechanisms leading to terminal differentiation of B cells into CD138+CD38++CD20− PCs in humans remain to be better characterized (Arpin et al., 1995; Litinskiy et al., 2002; Huggins et al., 2007).
In this study, we demonstrate that human Mϕs drive activated B cells to undergo proliferation and differentiation toward CD138+CD38++ terminally differentiated PCs, through the chemokine IP-10/CXCL10. The data reveal an amplification loop where B cell IL-6 induces Mϕs to secrete IP-10, which further boosts the B cell autocrine secretion of IL-6, leading to PC differentiation in a TI manner.
RESULTS
Tonsillar Mϕs induce Ig-secreting PCs
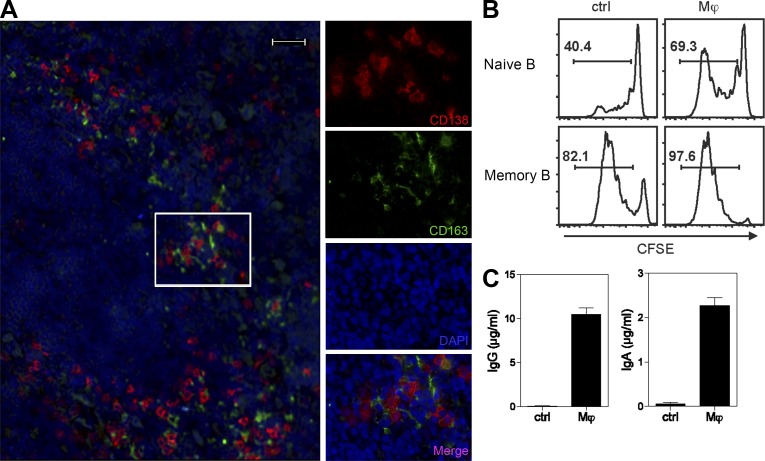
Analyzing the distribution of CD138+ cells in human tonsils by fluorescent immunohistology revealed that most CD138+ cells are surrounded by CD163+ resident Mϕs (Fig. 1 A and Video 1). Many CD138+ cells express intracytoplasmic Ig κ chain or λ chain, confirming that they are Ab-secreting cells (ASCs; not depicted). To establish the possible role of Mϕs in the induction of PC differentiation, human tonsillar Mϕs (CD163+HLA−DR+CD11c−) were sorted and co-cultured with autologous B cells that were preactivated with anti-Ig beads (to mimic BCR engagement by Ags) and in the presence of CpG (to mimic TLR9 engagement by microbial DNA), culture conditions which recapitulate in vivo B cell activation (Dullaers et al., 2009). Tonsillar Mϕs facilitated the proliferation of both naive (IgD+CD27−) and memory B (IgD−CD27+) cells (Fig. 1 B) and induced them to differentiate into CD138+ PCs (not depicted). Furthermore, tonsillar Mϕs significantly enhanced activated naive B cells to produce isotype-switched Igs such as IgG and IgA (Fig. 1 C).
Figure 1.
Human Mϕs induce terminal differentiation of PCs ex vivo. (A) Close localization of CD138+ PCs and CD163+ resident tonsillar Mϕs on a human tonsil section. Red indicates CD138-AF568, and green indicates CD163-FITC. Similar images were obtained from three different tonsils from different donors. Bar, 20 µm. (B) Sorted tonsillar naive (CD27−IgD+) or memory (CD27+IgD−) B cells were labeled with CFSE and cultured alone or co-cultured with autologous Mϕs for 6 d and analyzed for CFSE dilution by flow cytometry. (C) IgA and IgG production in the supernatants of 12-d co-culture of tonsillar naive B cells and Mϕs, as measured by ELISA. Data are representative of three independent experiments using tonsils from different donors. Mean ± SD of triplicate cultures is shown.
Monocyte-derived Mϕs induce differentiation of PCs and class switching
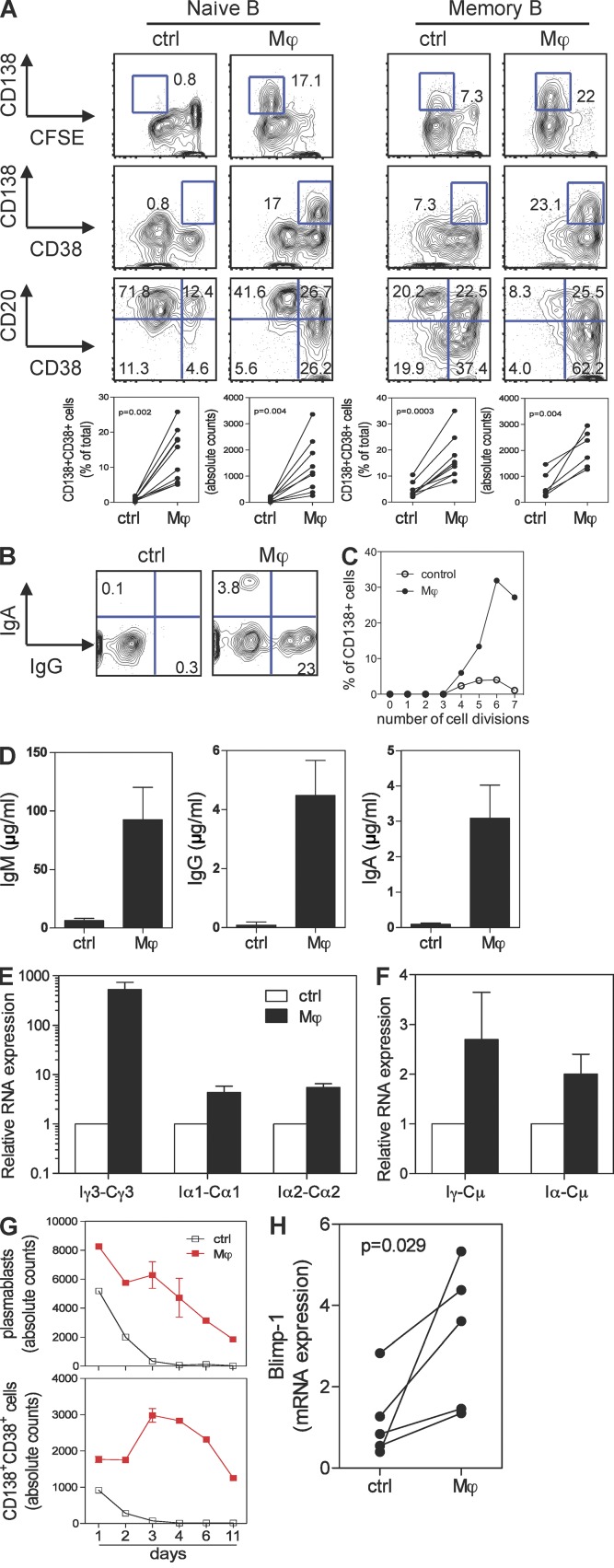
To help characterize the mechanism that Mϕs use to help PC differentiation, human CD163+ Mϕs were generated in vitro by culturing blood monocytes with M-CSF for 5 d (Verreck et al., 2004; Xu et al., 2006). Similar to their ex vivo counterparts, in vitro generated Mϕs efficiently induced activated B cells to differentiate into CD138+CD38++CD20− PCs (not depicted). Both naive and memory B cells became PCs upon exposure to Mϕs (Fig. 2 A). Naive B cells co-cultured with Mϕs underwent more proliferation, lost CD20, acquired CD38 (Fig. 2 A), and expressed both intracellular IgG (23%) and IgA (3.8%; Fig. 2 B). The detailed analysis of cell divisions (Hodgkin et al., 1996) revealed that B cell–Mϕ co-cultures had significantly more PCs per cell division than B cells cultured alone (Fig. 2 C). Cultured B cells secreted both IgG and IgA, in addition to IgM (Fig. 2 D). This was confirmed by the measurement of mature Ig transcripts (not depicted). In addition, Mϕs increased the rate of germline transcription of Iγ3-Cγ3, Iα1-Cα1, and Iα2-Cα2 (Fig. 2 E), as well as the generation of the switch circles Iγ-Cμ and Iα-Cμ in activated B cells (Fig. 2 F). Thus, Mϕs induce activated naive B cells to switch isotypes and to differentiate into PCs.
Figure 2.
Human Mϕs induce terminal differentiation of PCs from naive and memory B cells in vitro. (A) Sorted naive or memory B cells were co-cultured with autologous in vitro generated Mϕs or medium for 6 d and analyzed by flow cytometry for the expression of CD138, CD38, CD20, and CFSE. Both the percentage of CD138+CD38+ PCs and the absolute cell counts are shown. Each dot represents one independent experiment using a different donor. P-values were determined by two-sample Student’s t test. (B) Naive B cells co-cultured with autologous Mϕs for 6 d were stained intracellularly with Abs against IgA and IgG. (C) Percentage of CD138+ PCs per cell division was calculated based on FlowJo cell proliferation analysis. (D) IgM, IgA, and IgG production in the supernatants of co-culture of naive B cells and Mϕs, as measured by ELISA. Data are presented as mean ± SD from triplicate cultures and represent >10 different experiments using cells from different donors. (E and F) Naive B cells cultured with or without Mϕs were harvested on day 4 for isolation of mRNA and synthesis of cDNA. qPCR was performed to measure the expression of germline transcripts Iγ3-Cγ3, Iα1-Cα1, and Iα2-Cα2 (E) and switch circle transcripts Iγ-Cμ and Iα-Cμ (F). Expression was normalized to the amount of β-actin mRNA (for germline transcripts) or Im-Cm (for switch circles). B cells cultured alone were used as control for the relative expression. Data are mean ± SEM of three experiments using cells from different donors. (G) Sorted plasmablasts (CFSElowCD38+) were co-cultured with Mϕs for various days and analyzed for PC phenotype and counts. Mean ± SD of duplicate cultures is shown. (H) qPCR was performed to measure the expression of Blimp-1 on naive B cells co-cultured with Mϕs. P = 0.029, paired Student’s t test.
We next wondered whether Mϕs would provide survival signals to plasmablasts, as these cells die quickly in the absence of a survival niche (Ho et al., 1986; Smith et al., 1996). Accordingly, CFSElowCD38++CD20−CD138− plasmablasts were sorted from memory B cells that had been activated with anti-Ig and CpG for 5 d. In the absence of Mϕs, most plasmablasts died in culture by day 3, whereas those co-cultured with Mϕs survived and differentiated into PCs (Fig. 2 G) that secreted Ig (not depicted). As a control, DCs generated by culturing the same monocytes with GM-CSF and IL-4 did not help plasmablasts to become PCs (not depicted). Terminal differentiation of PCs is controlled by a transcriptional repressor, B lymphocyte–induced maturation protein 1 (Blimp-1; Shaffer et al., 2002; Nutt et al., 2007). Indeed, quantitative PCR indicated that B cells cultured with Mϕs express more Blimp-1 transcripts (Fig. 2 H). Therefore, Mϕs prolong survival of plasmablasts and induce terminal differentiation of PCs.
Mϕ-induced PC differentiation depends on IP-10 secretion
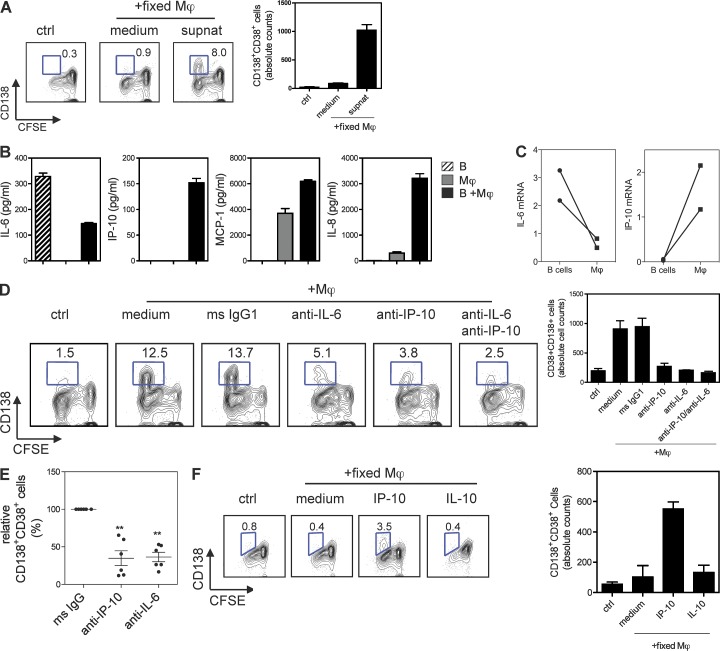
We next analyzed the potential contribution of soluble factors and/or cell–cell contact in the Mϕ-induced PC differentiation. Activated B cells co-cultured with fixed Mϕs (to prevent release of cytokines; Di Pucchio et al., 2008) did not generate PCs. However, further addition of supernatant from Mϕ–B cell co-cultures led to the generation of PCs (Fig. 3 A). This indicates that soluble factors able to induce PC differentiation were released by Mϕs and/or B cells during these co-cultures. APRIL and BAFF, released from APCs, have been shown to prolong B cell and plasmablast survival (Litinskiy et al., 2002; Craxton et al., 2003; Mackay et al., 2003). Mϕs do secrete measurable amounts of APRIL and BAFF (not depicted; Craxton et al., 2003). However, blocking BAFF and APRIL with Abs or a fusion protein, BCMA-Ig, did not effectively decrease the generation of PCs by Mϕs (not depicted). Thus, our data indicate that APRIL and BAFF may not be involved in the differentiation of PCs induced by Mϕs.
Figure 3.
Human Mϕs induce PC differentiation through IP-10. (A) Sorted naive B cells were co-cultured with fixed Mϕs or medium and/or with supernatants from Mϕ–B cell co-cultures. These were analyzed for PC phenotype and counts. (B) Luminex assay for the cytokines and chemokines in the supernatants from Mϕ–B cell co-cultures. (C) Naive B cells and Mϕs were cultured for 48 h. B cells and Mϕs were sorted for the extraction of total RNA, and qPCR was performed to measure the mRNA of IL-6 and IP-10. Data are relative expression of IL-6 or IP-10 normalized to the housekeeping gene GAPDH. Each dot represents data generated from a different donor. (D) Sorted naive B cells were co-cultured with autologous Mϕs in the presence of neutralizing Abs against IP-10, IL-6/IL-6R, or control mouse IgG1. The PC phenotype and absolute cell numbers for PCs are shown. (E) Summary of independent experiments using cells from different donors. Error bars indicate SEM of six different experiments. **, P = 0.001 (anti–IP-10) and P = 0.0002 (anti–IL-6), one-sample Student’s t test. (F) Sorted naive B cells were co-cultured with fixed Mϕs in the presence of recombinant human IP-10 or IL-10 and analyzed for PC counts. Data represent similar results from at least three independent experiments using different donors. (A, B, and D–F) Mean ± SD of duplicate (A, D, and F) or triplicate (B) cultures is shown.
Supernatants from co-cultures of B cells and Mϕs contained IP-10, IL-6, MCP-1, and IL-8. Activated B cells secreted only IL-6, whereas Mϕs alone produced MCP-1 and IL-8 (Fig. 3 B). Intracellular staining of co-cultured Mϕs and B cells revealed that B cells express IL-6, whereas Mϕs are the source of IP-10 (not depicted). This was further confirmed by quantitative PCR showing that sorted B cells in the co-cultures express more IL-6 messenger RNA (mRNA), whereas Mϕs express IP-10 mRNA (Fig. 3 C). Accordingly, neutralizing Abs against IP-10 and IL-6 significantly impaired the capacity of Mϕs to induce naive B cells to become PCs (Fig. 3, D and E). Conversely, anti–IL-8, anti–IL-10, and anti–MCP-1 neutralizing Abs did not affect the generation of PCs (not depicted). Addition of recombinant IP-10 could restore the ability of Mϕs to induce the PC differentiation that was abrogated by the anti–IP-10 Ab (not depicted), confirming that Mϕ-induced PC differentiation is IP-10 dependent. Furthermore, addition of recombinant IP-10 to fixed Mϕs induced activated naive B cells to become PCs (Fig. 3 F). When IP-10 signaling is inhibited by pertussis toxin, B cells lost their ability to become PCs induced by Mϕs (not depicted).
To distinguish the unique role of IP-10 in PC differentiation, we compared different soluble factors that have been shown to support B cell differentiation (Fairfax et al., 2008). Although many factors such as IL-6, IL-8, IL-10, and TNF are able to induce B cell proliferation and become CD38+ plasmablasts, IP-10 remains the only one to induce activated B cells to express CD138 (not depicted). Thus, IP-10 is able to induce the differentiation of activated B cells into PCs, revealing a novel functional property for this chemokine.
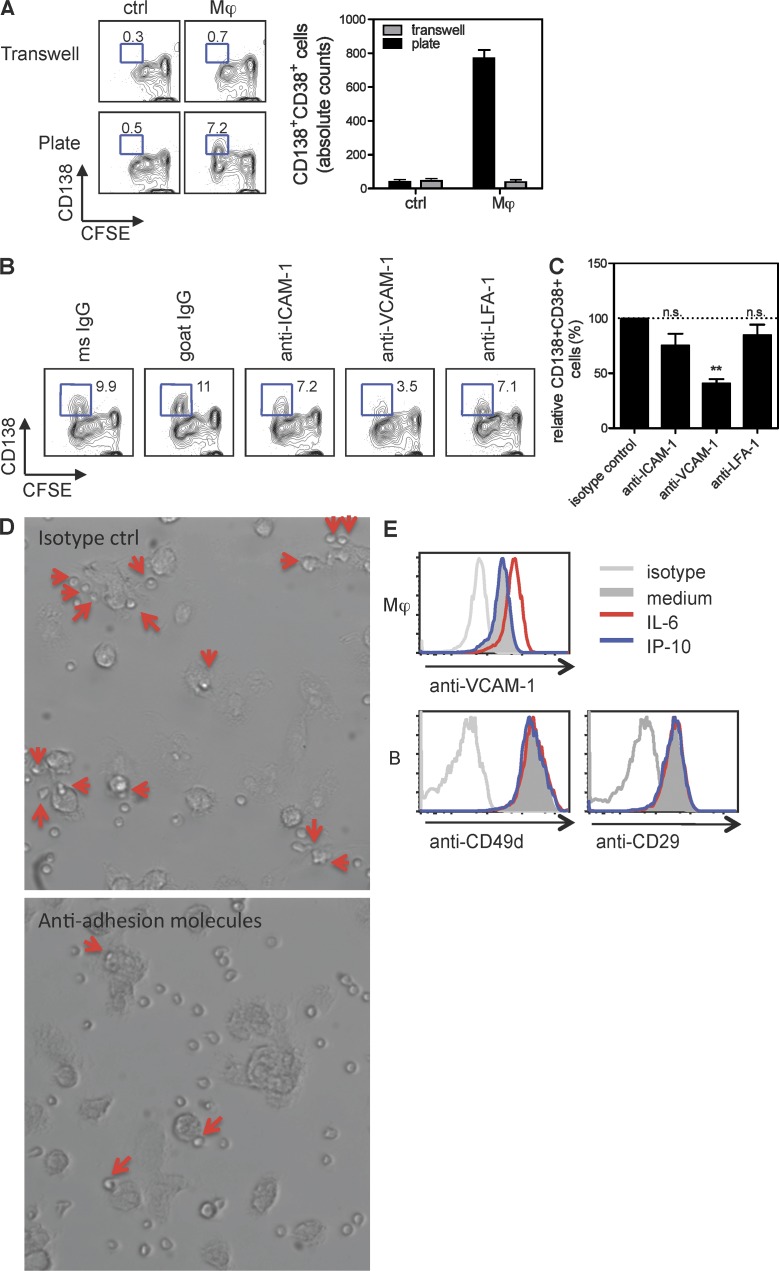
Mϕ-induced PC differentiation requires cell–cell contact via VCAM-1
To establish whether cell–cell contact is also necessary for Mϕs to induce PC differentiation, cell cultures were performed using dual-chamber vessels (transwells). Separating naive B cells from Mϕs in transwells prevented the generation of PCs (Fig. 4 A), indicating that cell–cell contact was required together with IP-10. Cell–cell contact can be mediated by a variety of adhesion molecules, including ICAM-1, LFA-1, and VCAM-1 (Springer, 1994), leading us to test whether Abs to these adhesion molecules could affect PC generation. As illustrated in Fig. 4 (B and C), anti–VCAM-1 Ab inhibited the generation of PCs by ∼50%, whereas other Abs were virtually ineffective. When anti–VCAM-1 was combined with anti–ICAM-1 and anti–LFA-1, PC differentiation was ∼80% blocked (not depicted), suggesting other adhesion molecules might also contribute to the B cell differentiation process. Blocking adhesion molecules prevented binding of B cells to Mϕs (Fig. 4 D), suggesting that Mϕs used adhesion molecules to tether B cells. Indeed, Mϕs failed to produce IP-10 when co-cultured with B cells in the presence of anti–VCAM-1/ICAM-1/LFA-1 (not depicted). This indicates that VCAM-1 engagement contributes predominantly to the Mϕ-dependent generation of PCs. Flow cytometry analysis revealed that VCAM-1 was expressed by Mϕs. Because the Mϕ–B cell co-cultures contained IL-6, IL-8, and IP-10, we tested whether any of these cytokines might induce VCAM-1 expression on Mϕs. Mϕs exposed to IL-6 up-regulated VCAM-1 expression (Fig. 4 E, top). As expected, all B cells expressed the dimeric ligand for VCAM-1 (CD49d and CD29; Fig. 4 E, bottom). Thus, Mϕs preferentially use the adhesion molecule VCAM-1 to tether activated B cells for the initiation of PC development.
Figure 4.
Mϕ-induced PC differentiation requires cell–cell contact through VCAM-1. (A) Sorted naive B cells were co-cultured with autologous Mϕs or medium for 6 d in 96-well plates or transwells and analyzed for PC phenotype and counts. Mean ± SD of duplicate cultures is shown. (B) Co-culture of naive B cells and Mϕs in the presence of 10 µg/ml anti–ICAM-1, anti–VCAM-1, and anti–LFA-1. (C) Data shown are mean ± SEM of relative PC counts from four independent experiments using cells from different donors. **, P = 0.0007, two-sample paired Student’s t test. (D) Total B cells were co-cultured with autologous Mϕs in the presence of mAb against VCAM-1, LFA-1, and ICAM-1 or isotype controls for 18 h. Images were taken using an Eclipse Ti microscope (Nikon) with a 20× objective. Arrows indicate B cells that are associated with Mϕs. (E, top) Mϕs were stimulated with 50 ng/ml IL-6 or 100 ng/ml IP-10 for 18 h and analyzed for the expression of VCAM-1 by flow cytometry. (bottom) Expression of CD49d and CD29 on B cells activated by IL-6 and IP-10 for 18 h.
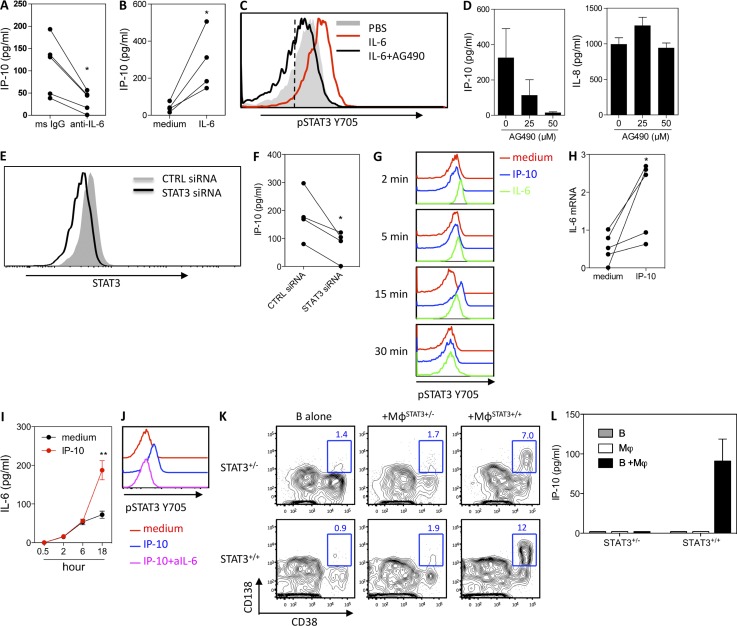
IP-10 production by Mϕs is dependent on IL-6 and STAT3 phosphorylation
We next examined whether B cells might contribute to the regulation of IP-10 production by Mϕs. Addition of anti–IL-6 Ab to the Mϕ–B cell co-cultures significantly reduced the production of IP-10 (Fig. 5 A). Conversely, Mϕs exposed to IL-6 secreted increased levels of IP-10 (Fig. 5 B). In line with an earlier study (Zhong et al., 1994), exposing Mϕs to IL-6 resulted in the phosphorylation of STAT3 at tyrosine 705 (Fig. 5 C). Blocking STAT3 with the STAT3 inhibitor AG490 impaired Mϕs to produce IP-10 without affecting the secretion of IL-8 (Fig. 5 D). To specifically address whether IP-10 production by Mϕs is dependent on STAT3, small interfering RNA (siRNA) was used to knock down STAT3 in Mϕs (Fig. 5 E). This resulted in an inhibition of IP-10 production by Mϕs exposed to IL-6 (Fig. 5 F). These data indicate that IL-6 enhances the production of IP-10 by Mϕs in a STAT3-dependent manner.
Figure 5.
STAT3-dependent regulation of IP-10 by Mϕs and B cells. (A) IP-10 production in the B cell–Mϕ co-cultures in the presence of neutralizing Abs against IL-6 and IL-6R. Data are from five independent experiments using cells from different donors. *, P = 0.0168, paired Student’s t test. (B) IP-10 production of Mϕs stimulated with 50 ng/ml recombinant IL-6 for 18 h. Data are from four independent experiments using cells from different donors. *, P = 0.0289, paired Student’s t test. (C) Mϕs cultured with IL-6 or in combination with 50 µM AG490 for 15 min and stained with phosphoflow Abs against pSTAT3 (Y705). The dashed line indicates the isotype control. (D) Production of IP-10 and IL-8 by Mϕs stimulated with recombinant IL-6 in the presence of AG490. (E) Mϕs were transfected with a STAT3 siRNA or control siRNA. Total STAT3 expression was measured at 72 h after transfection by flow cytometry. (F) Mϕs transfected with a STAT3 siRNA or control siRNA were stimulated with IL-6 for 48 h, and IP-10 was measured. Data are from four independent experiments using cells from different donors. *, P = 0.04, paired Student’s t test. (G) Total B cells were cultured with recombinant IL-6 or IP-10 for from 2 to 30 min and stained with phosphoflow Abs against pSTAT3 (Y705). Data are representative of at least three experiments. (H) Total B cells were cultured with or without 100 ng/ml IP-10 for 15 min, and total RNA was extracted for the measurement of IL-6 mRNA by qPCR. Data represent experiments using cells from five different donors. *, P = 0.04, paired Student’s t test. (I) Supernatants of B cells cultured with or without IP-10 were collected at various time points and measured for IL-6 by Luminex assay. Data are mean ± SEM from experiments using cells from three donors. **, P < 0.01, paired Student’s t test. (J) Total B cells were cultured with recombinant IP-10 or in combination with 10 µg/ml of an mAb against IL-6 for 15 min. Cells were stained with phosphoflow Abs against pSTAT3 (Y705). (K) Mϕs were generated in vitro from patients with hyper-IgE syndrome (STAT3+/−) or healthy donors (STAT3+/+). Sorted naive B cells (from patients or healthy donors) were preactivated with anti-BCR before being co-cultured with Mϕs in the presence of CpG for 6 d and analyzed for PC development. Data show the phenotype for CD38+CD138+ PCs. (L) Supernatant from a day 4 co-culture was collected for the measurement of IP-10. Data are representative of two independent experiments using cells from three patients. (D and L) Mean ± SD of duplicate (L) or triplicate (D) cultures is shown.
IP-10 amplifies IL-6 production by B cells
We next attempted to identify the signaling pathways that IP-10 uses to induce the differentiation of B cells. In the absence of a known IP-10 signaling pathway to guide us, we used phosphoflow and a panel of 57 different anti-phosphoprotein Abs (Table S1). Intriguingly, exposing B cells to IP-10 led to the phosphorylation of STAT3 at tyrosine 705 (Fig. 5 G) at a late time point (15 min). As expected, exposing B cells to IL-6 resulted in a rapid (2 min) phosphorylation of STAT3. As chemokine receptors are not known to be coupled to STAT proteins, we wondered whether IP-10 might act by activating the autocrine production of a B cell cytokine. Further analysis revealed that IP-10 amplified the autocrine production of IL-6 by activated B cells at mRNA level as early as 15 min (Fig. 5 H) and at detectable protein level later (Fig. 5 I). Addition of a neutralizing anti–IL-6 Ab abolished the IP-10–mediated STAT3 phosphorylation (Fig. 5 J), indicating that IP-10 signals through STAT3 indirectly by amplifying IL-6 in activated B cells.
To study the role of STAT3 in PC differentiation, we obtained naive B cells that have intrinsic heterozygous STAT3 mutation from patients suffering from hyper-IgE syndrome (Avery et al., 2010). STAT3-deficient naive B cells were not able to become PCs when co-cultured with STAT3-deficient Mϕs (Fig. 5 K), resulting from a failure of IP-10 production by Mϕs (Fig. 5 L). Although healthy Mϕs can drive STAT3-deficient B cells to become PCs, the differentiation was much reduced when compared with that observed with STAT3-intact B cells. STAT3-intact B cells failed to differentiate toward PCs when co-cultured with STAT3-deficient Mϕs (Fig. 5 K). Together, these data collectively suggest a crucial role of STAT3 signaling for both B cells and Mϕs in PC development.
IP-10 contributes to the induction of Ag-specific Abs in vivo
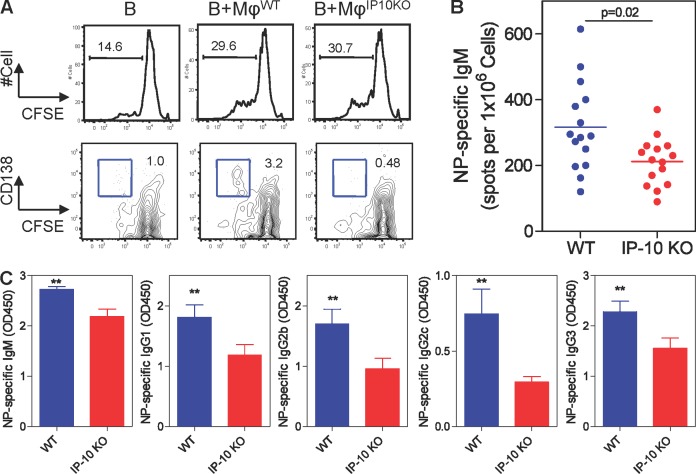
To further establish the role of IP-10 in the generation of PCs in vivo, we turned our attention to IP-10–KO mice, which show altered generation and trafficking of effector T cells (Dufour et al., 2002). In an initial attempt to recapitulate the function of IP-10 in mouse cells, B220+CD19+ B cells were isolated from the spleen and CD11b+F4/80+ Mϕs were sorted from splenocytes. Similar to the human B cells, mouse B cells failed to become CD138+ PCs when co-cultured with IP-10–deficient Mϕs (Fig. 6 A). There was no difference in total Ig titers in the serum between the WT and IP-10–KO mice before immunization (not depicted). 7 d after immunization with the TI Ag NP-Ficoll (Swanson et al., 2010), the frequency of total B cells and their subsets (including B1, follicular, and marginal zone B cells) in the spleen was similar between WT and IP-10–KO mice (not depicted). However, IP-10–KO animals showed a significantly lower frequency of NP-specific IgM-ASCs (mean = 212 versus 316 in WT; P = 0.02; Fig. 6 B). The IP-10–KO mice also produced significantly lower titers of NP-specific IgM, IgG1, IgG2b, IgG2c, and IgG3 (Fig. 6 C). These data suggest a contribution of IP-10 to Ag-specific PC differentiation in vivo.
Figure 6.
IP-10 contributes to the induction of Ag-specific Abs in vivo. (A) CFSE-labeled B cells and sorted Mϕs were co-cultured in the presence of 5 µg/ml Fab2′ anti-IgM and 50 nM CpG for 5 d and measured for the expression of CD138. Data are representative of two experiments of a total 6 mice per group. (B) C57BL/6 and IP-10–KO mice were immunized i.p. with 50 µg NP-Ficoll. Spleens were collected 7 d after immunization. Frequency of splenic NP-specific IgM ASCs was measured by ELISPOT. Data represent mean of 15 mice per group polled from three experiments. The p-value was determined by an unpaired Student’s t test. (C) Serum titers of NP-specific IgM, IgG1, IgG2b, IgG2c, and IgG3 were measured by ELISA. Serum was diluted in 1:180 for IgM and 1:60 for other Ig classes. Data represent mean ± SEM of nine mice per group pooled from two experiments. **, P < 0.01, a linear mixed model analysis.
DISCUSSION
Our studies in vitro and in vivo identify Mϕs as important players in the induction of PC terminal differentiation through the secretion of IP-10, a previously unrecognized mechanism (Charo and Ransohoff, 2006; Sallusto and Baggiolini, 2008). The IP-10 participates in PC development in an amplification loop where B cell–derived IL-6 induces Mϕs to secrete IP-10, which further boosts the B cell autocrine secretion of IL-6, leading to PC differentiation. To our knowledge, evidence that chemokines might play roles in cell differentiation other than in cell chemotaxis and trafficking remains scarce in spite of findings, such as the suppressor role played by Rantes, MIP-1α, and MIP-1β on the infection of CD4+ T cells by human immunodeficiency virus 1 (Cocchi et al., 1995) and role of CXCL12/SDF-1 as a survival factor for bone marrow PCs (Cassese et al., 2003).
Human resident Mϕs represent the most prevalent APCs in tissues, and these cells mostly maintain an antiinflammatory phenotype (Martinez et al., 2009). Ag-loaded Mϕs can present Ag either to T cells or B cells to initiate adaptive or humoral immunity, respectively (Gordon, 1986). Beneath the capsular layer in lymph nodes, a lining of resident Mϕs is available to encounter pathogens (Ochsenbein et al., 1999). This is particularly meaningful for large Ags such as particulates, immune complexes, and viruses, which are presented to B cells (Carrasco and Batista, 2007; Junt et al., 2007; Phan et al., 2009). Mϕs thus can control the retention and trafficking of B cells in the splenic marginal zone (Karlsson et al., 2003). The present study unravels a direct effect of resident Mϕs in the differentiation of PCs through IP-10 and a cell–cell contact interaction via VCAM-1 (Cocchi et al., 1995). Furthermore, B cell–derived IL-6 is able to up-regulate the expression of VCAM-1 on Mϕs, which attracts B cells that coexpress the dimeric ligands CD29 and CD49d. This is in line with an earlier study showing that VCAM-1 receptor–ligand interaction promotes membrane-bound Ag recognition and formation of an immune synapse (Carrasco and Batista, 2006). Our data therefore support the cross talk of Mϕs and B cells that culminates with the production of IP-10, possibly contributing to the initiation of the first wave of Ab protective immunity after pathogen capture by resident tissue Mϕs.
The upstream signal for IP-10 production by Mϕs is IL-6–induced STAT3 phosphorylation. IP-10 in turn further amplifies autocrine IL-6 production by activated B cells to sustain STAT3 signals. Note that the partial decrease of differentiation of PCs in STAT-deficient B cells could be explained by the possible contribution of residual STAT3 or other STATs such as STAT1 activated by IL-6. All together, our data support that STAT3 phosphorylation is an indispensable downstream signaling event in both Mϕs and B cells for the differentiation of IgM-, IgG-, and IgA-secreting PCs. This further contributes to explain why patients with hyper-IgE syndrome resulting from STAT3 mutations display deficient Ab responses (Avery et al., 2010). Our in vitro data are also supported by the partial inhibition (∼1.5-fold) of B cell differentiation observed in IP-10–deficient mice in response to TI Ags. Although it does not appear to be a considerably dramatic inhibition of PC differentiation, it is reminiscent of what is found both in mice and humans deficient in CD20 expression, which show a partial defect (∼1.8-fold) in Ab responses against TI Ag (Kuijpers et al., 2010). It cannot be excluded that defect in Ab response in IP-10–deficient mice results partially from impaired chemotaxis by any cell types that respond to IP-10. Thus, different molecules/pathways are likely to contribute to PC differentiation in the context of different triggers and tissue environments, and understanding the contribution of each of these pathways to human disease remains a complex challenge.
IP-10 is known to induce T cell trafficking and has been involved in several disease models, such as experimental autoimmune encephalomyelitis and mouse hepatitis virus infection, through binding to its ligand CXCR3 (Liu et al., 2005). In humans, IP-10 is elevated in the skin of patients with cutaneous lupus (Meller et al., 2005) and the kidney, central nerve system, and serum of systemic lupus erythematosus (SLE) patients (Narumi et al., 2000; Okamoto et al., 2004; Tsubaki et al., 2005). Furthermore, pre-PCs and PCs have been found concomitantly with IP-10 in these inflamed tissues. In healthy individuals, CXCR3 expression on B cells is limited to a fraction of naive and memory B cells, and its expression is induced by IFN-γ (Jones et al., 2000; Muehlinghaus et al., 2005). However, in certain diseases, such as active SLE, a subset of CD19high B cells enriched in autospecific Ig receptors expresses CXCR3 (Nicholas et al., 2008). Thus, excessive IP-10 and its signaling to CXCR3 on activated B cells might favor persistence of autoreactive PCs, suggesting that targeting IP-10 to interrupt PC development in autoimmune diseases such as SLE might be a valuable strategy to pursue.
In summary, our data reveal a novel role for Mϕs in the generation of PCs through the chemokine IP-10. Thus, APCs not only provide “signal 1” for BCR engagement on B cells (Harwood and Batista, 2010; Pierce and Liu, 2010), but further participate in a later stage of cell differentiation by providing an additional “signal 2.” Although interruption of this pathway might represent an efficient strategy to treat autoimmune diseases, enhancing Mϕ–B cell cross talk, for example by targeting Ag directly to Mϕs, might be considered to enhance vaccine-induced Ab responses.
MATERIALS AND METHODS
Human cells and cell cultures.
Minced tonsillar cells were from patients under 12 yr old who had tonsillectomies. Human tonsil collection and use have been approved by the Institutional Review Board of the Baylor Research Institute (approval no. 005-145). Total tonsillar B cells were isolated using the Human B Cell Enrichment kit (STEMCELL Technologies). Blood B cells were isolated from the lymphocyte-rich fractions by elutriation of PBMCs of healthy volunteers. Total B cells were purified from the same kit. Naive (CD27−IgD+) and memory (CD27+IgD−) cells were sorted on a FACSAria (BD), based on the gating on CD3−CD19+ B cells.
Tonsillar Mϕs were sorted (purity of 85–90%) from minced tonsillar cells, based on the expression of HLA-DR+CD163+CD11c−. Human in vitro Mϕs were generated from monocytes by culturing in complemented RPMI medium containing 10% FCS in the presence of 5 ng/ml M-CSF (R&D Systems) for 5 d, as described previously (Xu et al., 2006). In some experiments, Mϕs were fixed for 10 min in Cyto-Chex (Di Pucchio et al., 2008) and were washed three times in PBS before being cultured.
Naive B cells were cultured with anti-IgM–coated beads for 2 h at 4°C (500 ng/ml Immunobead rabbit anti–human IgM; Irvine Scientific). Memory B cells were stimulated with additional anti-IgA and anti-IgG. After labeling with CFSE (Molecule Probes), cells were cultured in complemented RPMI medium containing 10% FCS at 2 × 104/200 µl/well in the presence of 50 nM CpG (ODN2006; InvivoGen). The aforementioned stimuli form the basic in vitro culture conditions for B cells in this study, unless otherwise specifically indicated. Co-cultures of Mϕs and autologous B cells (Mϕ/B cell ratio of 1:2) were placed in the 96-well plates for 6 d before flow cytometry analysis. B cells cultured alone in the aforementioned basic culture condition were assigned as control. In some experiments, co-cultures of B cells and Mϕs were separated in transwell inserts (0.2-µM pores; Thermo Fisher Scientific).
Peripheral blood was collected from three patients with hyper-IgE syndrome with informed consent at the University of Texas Southwestern Medical Center and at Necker Medical School. These patients have heterozygous mutation of STAT3 at S116G, T708N, and K709E, respectively. PBMCs were isolated with Ficoll and then sorted for CD19+ B cells.
Mice and immunization.
C57BL/6 mice (WT) and IP-10–KO mice were purchased from the Jackson Laboratory and maintained within the animal facility at the Baylor Institute for Immunology Research. Mouse B cells were isolated from the spleen using a B cell negative selection kit (STEMCELL Technologies). The splenocytes that have gone through B cell selection were sorted for Mϕs based on the expression of CD11b+F4/80+. B cells were co-cultured with or without WT or IP-10–KO Mϕs in the presence of 5 µg/ml Fab2′ anti-IgM (SouthernBiotech) and 50 nM CpG for 5 d to measure PC development by flow cytometry.
Mice were immunized i.p. with 50 µg NP (4-hydroxy-3-nitrophenylacetic) hapten at NP28-Ficoll valences (Biosearch Technologies). Mice were sacrificed at day 7 after immunization, and splenocytes were collected. Sera were collected both at day 0 (preimmune) and day 7 (postimmune). The Institutional Animal Care and Use Committee of the Baylor Research Institute approved all experiments (no. A01-005).
Reagents and Abs.
The following fluorochrome-labeled Abs were used for flow cytometry and FACS sorting: anti-CD19, anti-CD27, anti-CD38, anti-CD138, anti-CD3, anti-CD20, anti-CD49d, and anti-CD29 from BD; anti–human IgD, IgM, IgG, and IgA from SouthernBiotech; and anti-BAFF and anti-APRIL from R&D Systems. Human neutralizing Abs including anti–IP-10, anti–IL-8, anti–MCP-1, anti–ICAM-1, anti–VCAM-1, anti–LFA-1, and isotype control Abs (mouse IgG1) were purchased from R&D Systems. The other neutralizing Abs, anti–IL-10 (clone 3D1.3F3) and anti–IL-6 (clone 30D2.1E11) and anti–IL-6R (clone 10F6.1E11; Jego et al., 2003) were generated in-house. These neutralizing Abs were used at 10 µg/ml. Recombinant IP-10 and IL-10 were purchased from R&D Systems. To block the effect of BAFF and APRIL, the following Abs or fusion proteins were used: mAb anti-BAFF, BCMA-muIg, and isotype controls (AnCell). Phosflow Ab pSTAT3Y705 was obtained from BD. A panel of all Phosflow Abs (from BD and Cell Signaling Technology) is listed in Table S1. For staining of mouse cells, goat anti–mouse B220, CD4, CD5, CD19, CD21, and CD23 were used (BD). CountBright Absolute Counting Beads (Invitrogen) were used to count cells during flow cytometry. The CellTrace CFSE Cell Proliferation kit (Invitrogen) was used to label B cells to track cell proliferation, and cell divisions were analyzed by the FlowJo proliferation platform (Tree Star).
ELISAs.
Sandwich ELISAs were performed to measure total IgM, IgG, and IgA in the culture supernatants, as described previously (Dullaers et al., 2009). A standard was from human reference serum (Bethyl Laboratories, Inc.) containing known amounts of the different Ig isotypes. Capturing and detection Abs were purchased from SouthernBiotech.
To measure the NP-specific Ab titers in mouse serum, plates were coated with 5 µg/ml NIP25-BSA (Biosearch Technologies) diluted in PBS. Detection was made by an alkaline phosphatase (AP)–conjugated goat anti–mouse isotype-specific Ab (SouthernBiotech) and followed by addition of AP substrate buffer consisting of 1 mg/ml 4-nitrophenyl phosphate disodium salt hexahydrate (Sigma-Aldrich).
BAFF and APRIL were measured in the culture supernatants from Mϕs stimulated with or without 50 ng/ml LPS (Sigma-Aldrich). The capture and detecting Abs and the standard are all from ZymoGenetics.
NP-specific ASC ELISPOT.
NP-specific ASCs were measured by ELISPOT, as described elsewhere (Swanson et al., 2010). In brief, nitrocellulose-bottomed 96-well Multiscreen HA filtration plates (Millipore) were coated with 5 µg/ml NIP25-BSA and incubated overnight at 4°C. Plates were washed with PBS and blocked with complete RPMI medium containing 10% FCS. Plates were washed again with PBS, and cell suspensions were added in volumes of 100 µl/well. After incubation for 3–4 h at 37°C in a humidified atmosphere containing 5% CO2, plates were thoroughly washed. AP-conjugated goat anti–mouse Abs diluted to 2 µg/ml in PBS containing 5% BSA was added, and the plates were incubated overnight at 4°C. After extensive washing, spots were developed at room temperature with 1 mg/ml 5-bromo-4-chloro-3-indolyl phosphate p-toluidine (Sigma-Aldrich) in diethanolamine buffer. Plates were washed and dried after optimal spot development, and spots representing individual ASCs were enumerated using a stereomicroscope (SZX12; Olympus) equipped with a vertical white light.
Cytokine multiplex analysis.
Cell culture supernatants were analyzed for a complete 22-plex, including IL-6, IL-10, IP-10, MCP-1, and IL-8, using the BeadLyte Cytokine Assay kit (Millipore) as per the manufacturer’s protocol. Fluorescence was analyzed with a Bio-Plex Luminex 100 XYP instrument (Luminex), and cytokine concentrations were calculated using Bio-Plex Manager 4.1 software with a five-parameter curve-fitting algorithm applied for standard curve calculations.
Tissue sections and immunostaining.
Tonsil tissue samples, obtained from patients under 12 yr old who had tonsillectomies, were frozen in OCT. A 6-µm-thick section was cut from the frozen tissues and was fixed with acetone and air dried before staining. The following Abs were used for staining: mouse anti–human CD163-FITC (BMA Biomedicals), mouse anti–human CD68 (BD), mouse anti–human CD138-Biotin (R&D Systems), SA-AF568, anti–FITC-488, goat anti–rabbit–Alexa Fluor 488, goat anti–mouse–Alexa Fluor 568, goat anti–rabbit–Alexa Fluor 488 (Molecular Probes), and goat anti–human κ light chain and rabbit anti–human λ chain (Abcam). The isotype control Abs are mouse IgG1 (R&D Systems), goat IgG-biotin (R&D Systems), and goat IgG-FITC (SouthernBiotech). DAPI (Molecular Probes) was used to counterstain the nuclei. Slides were imaged with a BX51 (Olympus) with Plan Apochromat 10×/0.4 and 40×/0.95 (for high magnification inserts) objectives, using a CoolSNAP HQ camera (Photometrics), and analyzed with MetaMorph software version 6.3 (Molecular Devices). Alternatively, slides were imaged with an SP5 confocal microscope (Leica) with a 63× APO objective and analyzed with Leica Application Suite 1.8.2 (for Video 1).
Conventional RT-PCR and quantitative real-time PCR (qPCR).
RNA was isolated from activated naive B cells cultured with or without Mϕs on day 4 using TRIZOL (Invitrogen), and cDNA was synthesized with the Reverse Transcription System (Promega). Conventional RT-PCR was performed for mature Ig transcripts VHDJH-CHμ, VHDJH-CHγ, VHDJH-CHα1, and VHDJH-CHα2. Quantitative real-time RT-PCR was used to measure germline transcripts Iγ3-Cγ3, Iα1-Cα1, and Iα2-Cα2, and switch circle transcripts Iγ-Cμ and Iα-Cμ. The primers for the aforementioned transcripts are as described earlier (Dullaers et al., 2009). Expression of Blimp-1 was measured by quantitative real-time RT-PCR using the following primer (Yan et al., 2007): forward, 5′-GACCGGCTACAAGACCCTTCCCTAC-3′; and reverse, 5′-ATGTGGCTTTTCTCCCGTGTGTACC-3′.
mRNA of IL-6 in B cells activated with IP-10 for 2 h were measured by real-time PCR. mRNA of IL-6 and IP-10 were measured in sorted B cells and Mϕs from a 2-d co-culture. The primers are the following: IL-6 forward, 5′-GGTACATCCTCGACGGCATCT-3′; and reverse, 5′-GTGCCTCTTTGCTGCTTTCAC-3′; and IP-10 forward, 5′-GTGGCATTCAAGGAGTACCTC-3′; and reverse, 5′-TGATGGCCTTCGATTCTGGATT-3′.
qPCR was performed on a Lightcycler 480 machine (Roche) using SYBR Green master mix (Roche). Expression was normalized to the amount of mRNA of reference genes (GAPDH, ACTB, or Im-Cm). The relative expression (RE) of a target gene was calculated using the following formula: REn = 2−(ΔCtn − ΔCt1), where ΔCtn (change in cycle threshold) is the cycle threshold of the test gene minus the cycle threshold of the reference gene, n is a specific sample, and 1 is the nontreatment sample.
Phosflow and intracellular staining.
For Phosflow staining, activated cells (2–30 min) were incubated with BD fixation buffer (BD) and then permeabilized with Phosflow Perm Buffer III (BD). pSTAT3 (Y705)-PE Ab was used to detect phosphorylated STAT3. For intracellular staining of cytokines (IL-6 and IP-10), Golgi transportation was blocked by GolgiStop and GolgiPlug (BD) in the last 4 h of culture and then fixed and permeabilized with Cytofix/Cytoperm Buffer (BD). Cells were stained with detecting Abs in Perm/Wash buffer (BD).
siRNA transfection.
Mϕs were transfected with siRNA with the Human Mϕ Nucleofector kit and Nucleofector II device (Amaxa). siRNA to target STAT3 and negative control siRNA (Thermo Fisher Scientific) were used at 0.2 nmol/2 × 106 cells/transfection. Cells were transferred at 6 h after transfection to the wells for stimulation with IL-6. IP-10 production was measured at 48 h of culture. The efficiency of STAT3 knockdown was measured for a total STAT3 expression by Phosflow staining 48 h after transfection.
Statistical analysis.
Two-sample, one-sample, and paired Student’s t tests were performed using Prism 4 software (GraphPad Software). A linear mixed model analysis with a random intercept (SAS software version 9.2) was used to test for differences of Ab titers between mouse types while accounting for repeated measures. Significant differences between experimental variables are noted with *, P < 0.05; or **, P < 0.01.
Online supplemental material.
Video 1 shows close localization of CD163+ Mϕs and CD138+ PCs. Table S1 is a list of phosphoflow Abs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20112142/DC1.
Supplementary Material
Acknowledgments
We thank Elizabeth Trahan and Sebastien Coquery in the FACS Core at the Baylor Institute for Immunology Research for cell sorting and Dr. Dan Su in the Baylor Tissue Bank for collecting human tonsils at Baylor University Medical Center. We are grateful to Florentina Marches for help with in vivo experiments, to Xiaohua Li and Indira Munagala for assistance with PCR, to Yoichiro Ohne for help with the phosphoflow assay, and to Ning Kang for assistance with Photoshop. We thank Drs. Carson Harrod and Gerard Zurawski for editing this manuscript. We also thank Dr. Michael Ramsay for supporting this study.
This study was supported by a pilot project of U19 (National Institutes of Health [NIH] #5U19AI057234). V. Pascual is supported by NIH grants AO50700-02 and AIO82715. W. Xu is the recipient of a Rubicon grant (#825.07.034) from The Netherlands Organization for Scientific Research (NWO).
W. Xu is currently an employee of Hoffmann–La Roche Inc. The authors have no other conflicting financial interests.
Footnotes
Abbreviations used:
- Ab
- antibody
- Ag
- antigen
- AP
- alkaline phosphatase
- ASC
- Ab-secreting cell
- Mϕ
- macrophage
- mRNA
- messenger RNA
- PC
- plasma cell
- qPCR
- quantitative real-time PCR
- siRNA
- small interfering RNA
- SLE
- systemic lupus erythematosus
- TD
- T cell dependent
- TI
- T cell independent
References
- Alugupalli K.R., Leong J.M., Woodland R.T., Muramatsu M., Honjo T., Gerstein R.M. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 21:379–390 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Arpin C., Déchanet J., Van Kooten C., Merville P., Grouard G., Brière F., Banchereau J., Liu Y.J. 1995. Generation of memory B cells and plasma cells in vitro. Science. 268:720–722 10.1126/science.7537388 [DOI] [PubMed] [Google Scholar]
- Avery D.T., Deenick E.K., Ma C.S., Suryani S., Simpson N., Chew G.Y., Chan T.D., Palendira U., Bustamante J., Boisson-Dupuis S., et al. 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207:155–171 10.1084/jem.20091706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista F.D., Harwood N.E. 2009. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 9:15–27 10.1038/nri2454 [DOI] [PubMed] [Google Scholar]
- Carrasco Y.R., Batista F.D. 2006. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 25:889–899 10.1038/sj.emboj.7600944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco Y.R., Batista F.D. 2007. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 27:160–171 10.1016/j.immuni.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Cassese G., Arce S., Hauser A.E., Lehnert K., Moewes B., Mostarac M., Muehlinghaus G., Szyska M., Radbruch A., Manz R.A. 2003. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171:1684–1690 [DOI] [PubMed] [Google Scholar]
- Charo I.F., Ransohoff R.M. 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354:610–621 10.1056/NEJMra052723 [DOI] [PubMed] [Google Scholar]
- Chilosi M., Adami F., Lestani M., Montagna L., Cimarosto L., Semenzato G., Pizzolo G., Menestrina F. 1999. CD138/syndecan-1: a useful immunohistochemical marker of normal and neoplastic plasma cells on routine trephine bone marrow biopsies. Mod. Pathol. 12:1101–1106 [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 270:1811–1815 10.1126/science.270.5243.1811 [DOI] [PubMed] [Google Scholar]
- Craxton A., Magaletti D., Ryan E.J., Clark E.A. 2003. Macrophage- and dendritic cell—dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 101:4464–4471 10.1182/blood-2002-10-3123 [DOI] [PubMed] [Google Scholar]
- Di Pucchio T., Chatterjee B., Smed-Sörensen A., Clayton S., Palazzo A., Montes M., Xue Y., Mellman I., Banchereau J., Connolly J.E. 2008. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 9:551–557 10.1038/ni.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour J.H., Dziejman M., Liu M.T., Leung J.H., Lane T.E., Luster A.D. 2002. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195–3204 [DOI] [PubMed] [Google Scholar]
- Dullaers M., Li D., Xue Y., Ni L., Gayet I., Morita R., Ueno H., Palucka K.A., Banchereau J., Oh S. 2009. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 30:120–129 10.1016/j.immuni.2008.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S., Honjo T. 2000. T-Independent immune response: new aspects of B cell biology. Science. 290:89–92 10.1126/science.290.5489.89 [DOI] [PubMed] [Google Scholar]
- Fairfax K.A., Kallies A., Nutt S.L., Tarlinton D.M. 2008. Plasma cell development: from B-cell subsets to long-term survival niches. Semin. Immunol. 20:49–58 10.1016/j.smim.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Fleire S.J., Goldman J.P., Carrasco Y.R., Weber M., Bray D., Batista F.D. 2006. B cell ligand discrimination through a spreading and contraction response. Science. 312:738–741 10.1126/science.1123940 [DOI] [PubMed] [Google Scholar]
- García de Vinuesa C., O’Leary P., Sze D.M., Toellner K.M., MacLennan I.C. 1999. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 29:1314–1323 [DOI] [PubMed] [Google Scholar]
- Gordon S. 1986. Biology of the macrophage. J. Cell Sci. Suppl. 4:267–286 [DOI] [PubMed] [Google Scholar]
- Harwood N.E., Batista F.D. 2010. Early events in B cell activation. Annu. Rev. Immunol. 28:185–210 10.1146/annurev-immunol-030409-101216 [DOI] [PubMed] [Google Scholar]
- Hiepe F., Dörner T., Hauser A.E., Hoyer B.F., Mei H., Radbruch A. 2011. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 7:170–178 10.1038/nrrheum.2011.1 [DOI] [PubMed] [Google Scholar]
- Ho F., Lortan J.E., MacLennan I.C., Khan M. 1986. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 16:1297–1301 10.1002/eji.1830161018 [DOI] [PubMed] [Google Scholar]
- Hodgkin P.D., Lee J.H., Lyons A.B. 1996. B cell differentiation and isotype switching is related to division cycle number. J. Exp. Med. 184:277–281 10.1084/jem.184.1.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M.C., Toellner K.M., Vinuesa C.G., Maclennan I.C. 2006. B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc. Natl. Acad. Sci. USA. 103:5905–5910 10.1073/pnas.0601502103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins J., Pellegrin T., Felgar R.E., Wei C., Brown M., Zheng B., Milner E.C., Bernstein S.H., Sanz I., Zand M.S. 2007. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 109:1611–1619 10.1182/blood-2006-03-008441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G., Palucka A.K., Blanck J.P., Chalouni C., Pascual V., Banchereau J. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 19:225–234 10.1016/S1074-7613(03)00208-5 [DOI] [PubMed] [Google Scholar]
- Jones D., Benjamin R.J., Shahsafaei A., Dorfman D.M. 2000. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 95:627–632 [PubMed] [Google Scholar]
- Junt T., Moseman E.A., Iannacone M., Massberg S., Lang P.A., Boes M., Fink K., Henrickson S.E., Shayakhmetov D.M., Di Paolo N.C., et al. 2007. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 450:110–114 10.1038/nature06287 [DOI] [PubMed] [Google Scholar]
- Karlsson M.C., Guinamard R., Bolland S., Sankala M., Steinman R.M., Ravetch J.V. 2003. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 198:333–340 10.1084/jem.20030684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T.W., Bende R.J., Baars P.A., Grummels A., Derks I.A., Dolman K.M., Beaumont T., Tedder T.F., van Noesel C.J., Eldering E., van Lier R.A. 2010. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J. Clin. Invest. 120:214–222 10.1172/JCI40231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel E.J., Butcher E.C. 2003. Plasma-cell homing. Nat. Rev. Immunol. 3:822–829 10.1038/nri1203 [DOI] [PubMed] [Google Scholar]
- Litinskiy M.B., Nardelli B., Hilbert D.M., He B., Schaffer A., Casali P., Cerutti A. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822–829 10.1038/ni829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Callahan M.K., Huang D., Ransohoff R.M. 2005. Chemokine receptor CXCR3: an unexpected enigma. Curr. Top. Dev. Biol. 68:149–181 10.1016/S0070-2153(05)68006-4 [DOI] [PubMed] [Google Scholar]
- Mackay F., Schneider P., Rennert P., Browning J. 2003. BAFF AND APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 21:231–264 10.1146/annurev.immunol.21.120601.141152 [DOI] [PubMed] [Google Scholar]
- Martinez F.O., Helming L., Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27:451–483 10.1146/annurev.immunol.021908.132532 [DOI] [PubMed] [Google Scholar]
- Medina F., Segundo C., Campos-Caro A., González-García I., Brieva J.A. 2002. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 99:2154–2161 10.1182/blood.V99.6.2154 [DOI] [PubMed] [Google Scholar]
- Meller S., Winterberg F., Gilliet M., Müller A., Lauceviciute I., Rieker J., Neumann N.J., Kubitza R., Gombert M., Bünemann E., et al. 2005. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus. Arthritis Rheum. 52:1504–1516 10.1002/art.21034 [DOI] [PubMed] [Google Scholar]
- Mond J.J., Lees A., Snapper C.M. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655–692 10.1146/annurev.iy.13.040195.003255 [DOI] [PubMed] [Google Scholar]
- Muehlinghaus G., Cigliano L., Huehn S., Peddinghaus A., Leyendeckers H., Hauser A.E., Hiepe F., Radbruch A., Arce S., Manz R.A. 2005. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 105:3965–3971 10.1182/blood-2004-08-2992 [DOI] [PubMed] [Google Scholar]
- Narumi S., Takeuchi T., Kobayashi Y., Konishi K. 2000. Serum levels of ifn-inducible PROTEIN-10 relating to the activity of systemic lupus erythematosus. Cytokine. 12:1561–1565 10.1006/cyto.2000.0757 [DOI] [PubMed] [Google Scholar]
- Nicholas M.W., Dooley M.A., Hogan S.L., Anolik J., Looney J., Sanz I., Clarke S.H. 2008. A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin. Immunol. 126:189–201 10.1016/j.clim.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt S.L., Fairfax K.A., Kallies A. 2007. BLIMP1 guides the fate of effector B and T cells. Nat. Rev. Immunol. 7:923–927 10.1038/nri2204 [DOI] [PubMed] [Google Scholar]
- Obukhanych T.V., Nussenzweig M.C. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203:305–310 10.1084/jem.20052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R.M. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- Okamoto H., Katsumata Y., Nishimura K., Kamatani N. 2004. Interferon-inducible protein 10/CXCL10 is increased in the cerebrospinal fluid of patients with central nervous system lupus. Arthritis Rheum. 50:3731–3732 10.1002/art.20598 [DOI] [PubMed] [Google Scholar]
- Oracki S.A., Walker J.A., Hibbs M.L., Corcoran L.M., Tarlinton D.M. 2010. Plasma cell development and survival. Immunol. Rev. 237:140–159 10.1111/j.1600-065X.2010.00940.x [DOI] [PubMed] [Google Scholar]
- Phan T.G., Green J.A., Gray E.E., Xu Y., Cyster J.G. 2009. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10:786–793 10.1038/ni.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce S.K., Liu W. 2010. The tipping points in the initiation of B cell signalling: how small changes make big differences. Nat. Rev. Immunol. 10:767–777 10.1038/nri2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Muehlinghaus G., Luger E.O., Inamine A., Smith K.G., Dörner T., Hiepe F. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6:741–750 10.1038/nri1886 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Baggiolini M. 2008. Chemokines and leukocyte traffic. Nat. Immunol. 9:949–952 10.1038/ni.f.214 [DOI] [PubMed] [Google Scholar]
- Shaffer A.L., Lin K.I., Kuo T.C., Yu X., Hurt E.M., Rosenwald A., Giltnane J.M., Yang L., Zhao H., Calame K., Staudt L.M. 2002. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 17:51–62 10.1016/S1074-7613(02)00335-7 [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M., Calame K. 2005. Regulation of plasma-cell development. Nat. Rev. Immunol. 5:230–242 10.1038/nri1572 [DOI] [PubMed] [Google Scholar]
- Smith K.G., Hewitson T.D., Nossal G.J., Tarlinton D.M. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26:444–448 10.1002/eji.1830260226 [DOI] [PubMed] [Google Scholar]
- Springer T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314 10.1016/0092-8674(94)90337-9 [DOI] [PubMed] [Google Scholar]
- Swanson C.L., Wilson T.J., Strauch P., Colonna M., Pelanda R., Torres R.M. 2010. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med. 207:1485–1500 10.1084/jem.20092695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki T., Takegawa S., Hanamoto H., Arita N., Kamogawa J., Yamamoto H., Takubo N., Nakata S., Yamada K., Yamamoto S., et al. 2005. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin. Exp. Immunol. 141:363–371 10.1111/j.1365-2249.2005.02850.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreck F.A., de Boer T., Langenberg D.M., Hoeve M.A., Kramer M., Vaisberg E., Kastelein R., Kolk A., de Waal-Malefyt R., Ottenhoff T.H. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA. 101:4560–4565 10.1073/pnas.0400983101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Roos A., Schlagwein N., Woltman A.M., Daha M.R., van Kooten C. 2006. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 107:4930–4937 10.1182/blood-2005-10-4144 [DOI] [PubMed] [Google Scholar]
- Yan J., Jiang J., Lim C.A., Wu Q., Ng H.H., Chin K.C. 2007. BLIMP1 regulates cell growth through repression of p53 transcription. Proc. Natl. Acad. Sci. USA. 104:1841–1846 10.1073/pnas.0605562104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J.E., Jr 1994. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 264:95–98 10.1126/science.8140422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.